File
advertisement

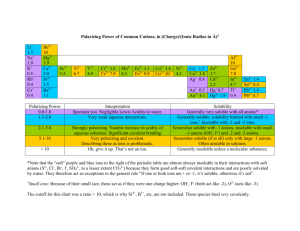
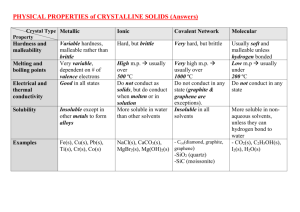
Metallic Substance type Metal element Examples Na, Fe, Cu Structure Giant lattice of positive ions in a ‘sea’ of electrons Bonding Attraction of delocalised electrons for positive ions binds structure together by strong metallic bonds. Properties Melting and boiling points High m.pt. Covalent Network Non-metals in Group 3 and 4 or its compound Ionic Covalent Molecular Usually metal/non-metal compound(a compound with a relatively large difference in electronegativity NaCl, CaO, K2SO4 Non-metal elements or usually non-metal/nonmetal compound Diamond, graphite, SiC, B Giant lattice of Giant lattice of covalently positive ions bonded atoms and negative ions Atoms are Attraction of linked positive ions for throughout the negative ions whole gives strong structure by ionic bond. strong covalent bonds. Very high m.pt. High m.pt. I2(s), S8(s), C8H18(l), HCl(g), P4(s) Discrete molecules Strong covalent bonds hold atoms together within the separate molecules held together by weak intermolecular forces (London dispersion forces or hydrogen bonds). Low m.pt. and b.pt. (hydrogen bonding raises m.pt. and b.pt. compared with London dispersion forces) Solids, liquids or gases State at room temp. Usually solid Solid Electrical conductivity Good conductors Nonwhen solid or liquid conductors (graphite is an exception) Solubility Insoluble in polar and non-polar solvents Solid Non-conductors when solid, good conductors when molten or in aqueous solution – electrolytes. Insoluble in all Soluble in polar solvents solvents, insoluble in non-polar solvents Non-conductors in all states (a few e.g. HCl(g) react with water to form electrolytes). Generally insoluble in polar solvents but soluble in non-polar solvents.