Review Sheet
advertisement

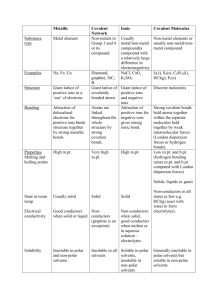
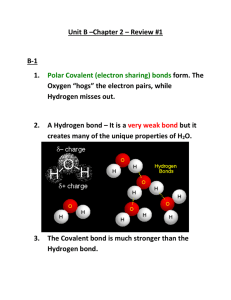
CP Biology Name Date Covalent and Ionic Compounds Dissolving in Water Pd 1) Given the following beaker of water, draw what it would happen if potassium chloride (KCl) was added. Potassium chloride is an ionic compound composed of K+ and Cl-. 2) Given the following beaker of water, draw what it would happen if ribose sugar (C5H10O5) was added. Ribose sugar is a polar covalent compound similar to glucose. 3) What property does water have that allows it to dissolve the solutes above? _________________________________________________________________________________ 4) What property do the solutes (KCl and C5H10O5) have that allow them to be dissolved in water? _________________________________________________________________________________ 5) Which of the beakers above (1, 2 or both) shows dissociation? _______________ 6) What is dissociation? _________________________________________________________________________________ pH HW 1. What happens to an ionic compound when it is placed in water? 2. What do we call a compound that releases H+ ions in solution? 3. What ions does HCl dissociate into? 4. What is a base? 5. a. b. c. d. Which of the following is true regarding a neutral compound? in solution the concentration of H+ ions is the same as the concentration of OH- ions. it has a pH = 7 pure water is an example all of these 6. A solution with a pH of 8 contains __?__ more H+ ions than a solution with a pH of 12. a. 1 b. 4 c. 104 d. 10,000 7. Which solution contains more OH- ions? a. pH 8.2 b. pH 8.3 8. If we mixed KCl (a common salt) with pure water, do you predict the resulting solution would have a pH above, equal to, or below 7? Explain. 9. What does the pH scale measure? 10. Label the following solutions as acid, base or neutral. _________pH 1 _________pH 8.9 _________pH 14 _________pH 3.2 _________pH 7 11. Why is pure water neutral? 12. On the pH scale below, label the arrows on either side of "Neutral" to indicate which direction is increasingly acidic and which is increasingly basic. 13. Name any two solutions that have more H+ ions than OH– ions. 14. Name any two solutions that have more OH– ions than H+ ions.