click here now
advertisement

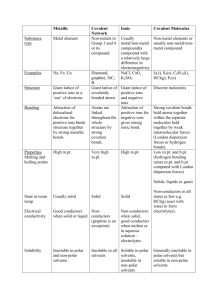
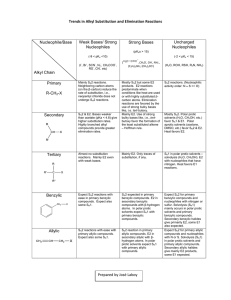
Amyloid β on Nobel Metals: surface-enhanced Raman spectroscopy investigates the structure, while AFM investigates the function Debanjan Bhowmik1, Christina MacLaughlin2, Shell Ip2, Suman Nag1, Bidyut Sarkar1, C. Muralidharan1, Gilbert Walker2 and Sudipta Maiti1* 1 Department of Chemistry, TIFR, Mumbai Department of Chemistry, Lash Miller Laboratories, UT, Toronto *maiti@tifr.res.in 2 Medicinal activity of a molecule is highly dependent on the presence of a biologically important unit. We have designed two keto-tetrahydrocarbazole derivatives 2-((9-methyl-1-oxo-2,3,4,9-tetrahydro-1H-carbazol-6-yl)oxy)acetic acid and 2-((7-methoxy-9-methyl-1-oxo-2,3,4,9-tetrahydro-1H-carbazol-6yl)oxy)acetic acid, both of which are efficient fluorophores. We have used rigorous spectroscopic investigations to elucidate the photophysical properties of the compounds in different solvents. Absorption maxima of the compounds have changed to a small extent in solvents with largely different empirical polarity parameters [ET(30)]. The fluorescence emission maxima of the compounds, on the contrary, have undergone red shift with increasing [ET(30)] of the solvents. Protic solvents were especially influencial in perturbing the excited states of the fluorophores. Calculations of dipole moment, free energy of solvation, reorganization energy, solvatochromic comparison method have been used to describe the excited state properties of the compounds. Binary solvent mixtures were used to demonstrate the dielectric enrichment of protic solvents in comparison with aprotic solvents. Since their discovery, flavins have been recognized to play pivotal role in versatile biochemical redox reactions as they functions as both electrofiles and nucleophiles inside the complex biological enviroment. In this presentation we decipher the reaction pathways of Riboflavin (Vitamin B2) in presence of organic bases (N, N- Dimethylaniline and Triethylamine) in both homogeneous and heterogeneous media. References: [1] Ellis, R. J. Trends Biochem. Sci. 2001, 26, 597 – 604. [2] Minton, A. P. J. Biol. Chem. 2001, 276, 10577-10580. [3] Hall, Damien.; Minton, Allen P. Biochimica et Biophysica Acta.2003, 1649, 127139. [4] Schlesinger, A.P.; Wang, Y.; Tadeo, X.; Millet, O.; Pielak, G.J. J. Am. Chem. Soc. 2011, 133, 8082 – 8085. Abstract format Single page abstracts with a maximum of 300 words should be submitted in Microsoft Word format (.doc or .docx) The font should be Myriad or Myriad Pro or Calibri Title should be in 12pt bold, upper-lower case letters and centered. This should be followed by authors' names (9 pt normal) and affiliation (8pt, italics)centered . The abstract text should be (9pt) justified. A maximum of one figure/graph may be added if required (page size should not exceed one page). References will be in 8 pt, left aligned. Be noted that only .doc/.docx file can be uploaded.