Ch4 Questions
advertisement

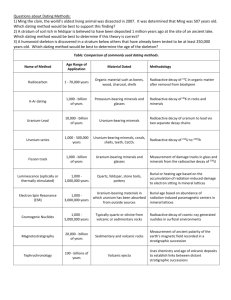
Ch. 4 Review Fig. 4.2, 4.3, 4.5, 4.7b, 4.11 Refer to quizzes given in class Terms: Pg. 75: absolute dating, alpha decay, beta decay, carbon 14 dating, catastrophism, daughter element, half life, parent element, relative dating, principles for relative dating (below) Review Questions Pg. 75: 2, 3, 6, 7, 9, 10, 12, 14 (Answers page 430) Concepts: Relative Dating principles: cross cutting relationships, lateral continuity, horizontality, superposition, fossil succession, inclusions tree ring dating Absolute Dating radioactive decay of isotopes: rate of decay is same for all radioactive isotopes. The number of years for half of a radioactive substance or “parent” to decay to stable “daughter” produces is the half-life for a particular isotope. For example, Uranium 238 – Lead 206 has a half life of 4.5 billion years (by). Uranium 235 – Lead 207 has a half life of 704 million years (my). Half lives (the actual number of years) are different for every radioactive parent/daughter pair. – decay curve – be able to determine the number of half lives on the graph. calculation of age - alpha decay – two protons and two neutrons are emitted, lowering the atomic mass by four mass units an alpha decay results in two fewer protons, the atomic number goes down by 2, producing a new element -beta decay – an electron is lost from a neutron. There is no mass change. However, the neutron loses a negative charge and becomes positive, making it now a proton. The atomic number goes up by 1, making a new element. catastrophism (young earth, Bible based time scale in thousands of years)– principle of uniformitarianism (old earth, millions of years or more) 1.Placing geologic events in a chronological order from their position in the rock record is A. neptunism B. uniformitarianism C. relative dating D. absolute dating 2.Assigning a specific date to a rock, which is measured in years before present, is known as A. relative dating B. absolute dating C. superposition D. catastrophism 3.Radiometric dating is based upon A. the presence of fossils B. cross cutting relationships C. spontaneous decay of radioactive elements D. superposition 4.The geologic time scale that we use today is based upon A. absolute time and relative time B. absolute time and geographic locations C. relative time and obsolete time D. relative time and fossil lithologies 5. The observation that in an undisturbed section of sedimentary rock the oldest is on the bottom and the youngest is on the top is A. the principle of cross-cutting relations B. the principle of lateral continuity C. the principle of inclusions D. the principle of superposition 6. If an igneous rock cuts across a sedimentary rock, we know that the igneous rock is A. older than the sedimentary rock B. younger than the sedimentary rock C. the same age as the sedimentary rock D. a minimum of 1.2 million years old 7. The observation that sediment is deposited in essentially flat layers led to A. the principle of cross-cutting relations B. the principle of lateral continuity C. the principle of original horizontality D. the principle of superposition 8. The concepts that eventually formed the basis for uniformitarianism stated that A. the past history of the Earth can be explained by present-day processes B. Earth’s rocks are precipitated in ocean basins C. the Earth is approximately 10,000 years old D. major events, such as widespread flooding, are responsible for the Earth’s surface E. All of the above 9. Radioactive Na24 decays with a half-life of 15 hours. Starting with 1000 atoms of this element, how many atoms would exist after 45 hours? A. 500 B. 250 C. 125 D. 996 E. no Na24 would remain 10. If a radioactive element has a half-life of 4,000,000 years, the amount of parent material remaining after 12,000,000 years of decay will be this fraction of the original amount: A. 1/32 B. l/16 C. 1/8 D. 1/4 11. The atomic number of an element is the same as the number of A. electrons in the nucleus B. neutrons in the nucleus C. protons in the nucleus D. electrons in the outer shell E. protons and neutrons in the nucleus 12. Alpha decay is A. the loss of a proton and a neutron B. the loss of a proton and capture of an electron C. the loss of two protons and two neutrons D. the loss of an electron from a neutron E. the capture of an electron 13.Beta decay is A. the loss of a proton and a neutron B. the loss of a proton and capture of an electron C. the loss of two protons and two neutrons D. the loss of an electron from a neutron E. the capture of an electron 14. The half-life of a radioactive isotope is A. half the amount of time required for the isotope to decay B. half the duration of a radioactive isotope’s existence C. half of the isotope’s decay rate D. the time necessary for half of the radioactive parent atoms to decay to daughter atoms 15. If an isotope has a half-life of 50 million years, and 1/8 of the remaining sample consists of the parent isotope, the age of the rock from which the sample was taken is A. 400 million years B. 300 million years C. 150 million years D. 100 million years 16. In the field, you encounter layers of sedimentary rock through which a dike of igneous granite intrudes. The granite is dated radiometrically to be 28.7 million years. How old is the sedimentary rock? A. younger than 28.7 million years B. 28.7 million years C. older than 28.7 million years D. 40.2 million years 17. If a sandstone contains inclusions of an underlying granite, we can infer that A. the sandstone is older than the granite B. the relative age of the sandstone and granite cannot be determined C. the granite intruded into the sandstone D. the sandstone is younger than the granite 18. Complete the following table for radioactive decay of any element: % Parent remaining 50 % Daughter Accumulated # of Half Lives Elapsed 75 12.5 93.75 3.13 Note: % Parent and % Daughter must add to 100% 19. Complete the following table for commonly used radioactive decay pairs. USE THE DATA IN YOUR TEXTBOOK. Radioactive Parent Element Uranium 238 (U238) Stable Daughter Element Lead 207 (Pb207) Carbon 14 (C14) 20. In the chart of radioactive decay shown below: # of years for one half life to occur how many half-lives have elapsed by time “a” and “b”? (a) ____________________ (b) ____________________ Parent 21. An igneous rock contains a zircon mineral with 12.5% U235 and 87.5% of Pb207. What is the absolute age of the rock? A. There is insufficient information to calculate this. B. approximately 705 my C. Less than 705 my D. approximately 2.1 by 22. A fossil tree contains 50% C14 and 50% N14. What is the absolute age of the fossil tree? A. There is insufficient information to calculate this. B. 2,865y C. 5,730y D. 11,460y Use the block diagram below to answer the following questions. Note that all layers are sedimentary EXCEPT for K and L which are igneous. Event M is a fault. The wavy black lines represent unconformities. 23. Write the letters from oldest to youngest including all layers, intrusions, and events. ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ 24. Fine two unconformities. Describe between which two layers the unconformities each occur. List the type of unconformity. Unconformitiy 1 between __________and _______________ _________________________________ Type Unconformity 2 between __________and _______________ Type_________________________________ 25. Is Igneous intrusion older than layer C or younger than layer C? How can you tell? ___________________________ ___________________________________________________________________ ___________________________