Section 3 Assessment
Section 3 Assessment
Page 334
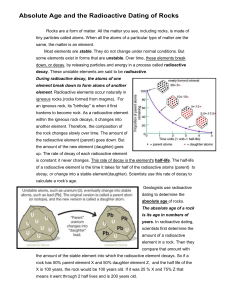
Radioactive Dating
1a Define radioactive decay
• Radioactive decay is the process by which unstable atoms release particles and energy in an effort to become stable.
1b How does the composition of a rock containing a radioactive element change over time?
• The amount of the radioactive element decreases.
• The amount of the stable element increases.
How is the rate of radioactive decay like the ticking of a clock?
• The rate of decay is constant – it never changes. It doesn’t speed up or slow down.
What method do geologists use to determine the absolute age of a rock?
• Geologists use radioactive dating to determine the absolute age of igneous rock.
Why is it difficult to determine the absolute age of sedimentary rock?
• The particles in sedimentary rock are made up of substances of different ages.
Problem Solving
• A geologist finds a fossil in a layer of sedimentary rock that lies in between two igneous extrusions. How could the geologist determine the age of the fossil?
• The scientist could use radioactive dating to find the ages of the two igneous extrusions.
The age of the sedimentary rock would be somewhere in between the two ages.
Math Practice
• What percentage of a radioactive element will remain after 7 half lives?
• ½ x ½ x ½ x ½ x ½ x ½ x ½
• 1/128
• .0078125
• .78125%