BTPR_1500_sm_SuppInfo
advertisement

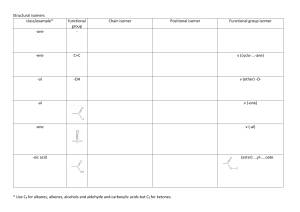
Supplementary Material Supplementary Figure 1 The effect of biomass concentration on specific activity. Supplementary Figure 2 Specific activities achieved using cells grown to a similar biomass concentration in the eight media formulations tested. Oxocan-2-one1 Cycloheptanone (1.00 g, 8.93 mmol) and m-chloroperbenzoic acid (0.168 g, 9.74 mmol) in dichloromethane (10 ml) were stirred for 24 h at rt. The mixture was washed with saturated sodium carbonate solution (3 x 10 ml) and saturated sodium chloride solution (10 ml). The organic layer was dried (Na2SO4) and concentrated in vacuo.2 The crude product was purified by flash silica chromatography (ethyl acetate:hexane, 1:1) to give oxocan-2-one as a colourless oil (0.620 g, 54%). nmax(neat)/cm–1 2931, 1725; 1H NMR (600 MHz; CDCl3) d 1.58–1.66 (4H, m) 1.74–1.90 (4H, m) 2.54 (2H, t, J 6.4, CH2CO) 4.34 (2H, t, J 5.7, CH2O); 13C NMR (150 MHz; CDCl3) d 24.0, 25.9, 28.4, 31.0, 31.4, 68.0 (CH2O), 176.9 (C=O); m/z (HRCI) found [MH]+ 129.09101. C7H13O2 requires 129.09155. GC retention time: 14.8 min. 2-Oxa-bicyclo[3.2.1]octan-3-one3,4 After a baffled shake flask fermentation equivalent to that described in section 2.3.3 with racemic norcamphor as a substrate, a whole cell solution (200 ml) was centrifuged and the supernatant was extracted with ethyl acetate (200 ml). The organic layer was dried (Na2SO4) and concentrated in vacuo. The crude product was purified by flash silica chromatography (ethyl acetate:hexane, 1:1) to give the major isomer 2-oxa-bicyclo[3.2.1]octan-3-one and minor isomer 3-oxabicyclo[3.2.1]octan-2-one in a ratio of 15:1, as a colourless oil in 43% isolated yield (50 mg). nmax(neat)/cm–1 2950, 1737; 1H NMR (600 MHz; CDCl3) (major isomer) d 1.61–1.75 (2H, m) 1.88–2.00 (3H, m) 2.08–2.20 (1H, m) 2.42– 2.52 (1H, m) 2.52–2.57 (1H, m) 2.68–2.74 (1H, m) 4.86 (1H, m, CHOCO); 13C NMR (150 MHz; CDCl3) (major isomer) d 29.1, 31.9, 32.5, 35.8, 40.7, 81.0 (CHO), 170.9 (C=O); m/z (HREI) found [M]+ 126.06814. C7H10O2 requires 126.06808. GC retention time (major isomer): 10.5 min. References 1. Hasegawa Y, Hamano K, Obata H, Tokuyama T. Microbial degradation of cycloheptanone. Agricultural and Biological Chemistry. 1982;45(5):1139-1143. 2. Harikrishna M, Mohan HR, Dubey PK, Subbaraju GV. Synthesis of 2-Normisoprost, Methyl 6(3-Hydroxy-2-((E)-4-hydroxy-4-methyloct-1-enyl)-5-oxocyclopentyl)hexanoate. Synthetic Commun. 2009:39:2763-2775. 3. Abril O, Ryerson CC, Walsh C, Whitesides GM. Enzymatic Baeyer-Villiger type oxidations of ketones catalyzed by cyclohexanone oxygenase. Bioorganic Chemistry. 1989;17(1):41-52. 4. Mihovilovic M, Kapitán P, Kapitánová P. Regiodivergent Baeyer–Villiger Oxidation of Fused Ketones by Recombinant Whole-Cell Biocatalysts. ChemSusChem. 2008;1(1-2):143-148.