Supporting information 3-Aminopropyl
advertisement

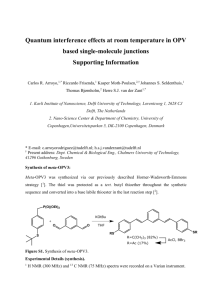
Supporting information 3-Aminopropyl-triethoxysilane grafted on graphene oxide: A highly efficient and recyclable catalyst for Knoevenagel condensation Jie Huang, Shunmin Ding, Weiming Xiao, Yadan Peng, Shengjun Deng, Ning Zhang* Department of Chemistry, Nanchang University, Nanchang, 330031, P. R. China * Corresponding author. E-mail: nzhang.ncu@163.com; Captions 1. Synthesis of GO. 2. Acid oxidation of CNTs. 3. Synthesis of SiO2-APTS, MCM-41-APTS and CNTs-APTS. 4. Selected 1HNMR data for typical compounds. Synthesis of GO [S1] Graphite powder (3 g, 8000 mesh) was put into an 80 °C solution of 98% H2SO4 (12 mL), K2S2O8 (2.5 g), and P2O5 (2.5 g). The mixture was kept at 80 °C for 4.5 h using oil bath. Successively, the mixture was cooled to room temperature and diluted with 500 mL of deionized water and left overnight. Then, the mixture was filtered and washed with de-ionized water. The product was dried under ambient condition overnight. This pre-oxidized graphite was then subjected to oxidation by Hummers’ method described as follows. Pretreated graphite powder was put into cold (0 °C) 98% H2SO4 (120 mL). Then, KMnO4 (15 g) was added gradually under stirring and the temperature was kept to be below 20 °C. Successively, the mixture was stirred at 35 °C for 2 h, and then diluted with de-ionized water (250 mL), and keep the temperature below 50 °CAnd the mixture stirred for 2 h, and then added additional 700 mL of de-ionized water, and 20 mL of 30% H2O2 was added to the mixture. The mixture was filtered and washed with 10% HCl aqueous solution (1 L) to remove metal ions followed by 2 L of de-ionized water to remove the acid. The resulting solid was dried under vacuum at 40 °C for 48 h. Acid oxidation of CNT: The CNT (1.2 g) samples were spurified by reflux in a mixture of 3 mL HNO3 and 15 mL H2SO4 at 80 °C for 1 h, and then cooled to room temperature. The obtianed samples were filtered and washed with DI water until the filtrate pH reached neutral, and dried overnight under vacuum at 60 °C for 48 h. Synthesis of SiO2-APTS, MCM-41-APTS and CNT-APTS A solution of 2.2 g (10 mmol) 3-Aminopropyl-triethoxysilane in 9 mL dry chloroform was added to a suspension of 1 g SiO2 (or MCM-41[S2] or CNT ) in 160 mL of dry toluene. The mixture was stirred at 100 °C for 72 h. Then the solid was filtered and washed by dry chloroform (6 × 20 mL) and ethanol (3 × 10 mL), and dried in vacuum at 80 °C for 48 h. Selected 1HNMR data for typical compounds Ethyl (E)-2-cyano-3-phenyl-2-propenoate [30] (Table 1, entry 1). Melting point 46-49 °C, H NMR (601 MHz, CDCl3) δ 8.24 (s, 1H), 7.98 (d, J = 7.6 Hz, 2H), 7.52 (dt, J = 15.2, 7.3 1 Hz, 3H), 4.37 (q, J = 7.1 Hz, 2H), 1.56 (s, 2H), 1.38 (t, J = 7.1 Hz, 3H). Ethyl (E)-2-cyano-3-(4-nitrophenyl)-2-propenoate [30] (Table 1, entry 2). Melting point 163-166 °C, 1H NMR (601 MHz, DMSO) δ 8.56 (s, 1H), 8.41 (d, J = 8.9 Hz, 2H), 8.25 (d, J = 8.5 Hz, 2H), 4.35 (q, J = 7.1 Hz, 2H), 1.33 (t, J = 7.1 Hz, 3H). Ethyl (E)-2-cyano-3-(4-chlorophenyl)-2-propenoate [25] (Table 1, entry 3). Melting point 88-90 °C, 1H NMR (601 MHz, DMSO) δ 8.42 (s, 1H), 8.08 (d, J = 8.5 Hz, 2H), 7.69 (d, J = 8.6 Hz, 2H), 4.33 (q, J = 7.1 Hz, 2H), 1.31 (t, J = 7.1 Hz, 3H). Ethyl (E)-2-cyano-3-(4-methylphenyl)-2-propenoate [28] (Table 1, entry 4). Melting point 91-93 °C, 1H NMR (601 MHz, CDCl3) δ 8.20 (s, 1H), 7.88 (d, J = 7.9 Hz, 2H), 7.29 (d, J = 7.9 Hz, 2H), 4.36 (q, J = 7.1 Hz, 2H), 2.42 (s, 3H), 1.38 (t, J = 7.1 Hz, 3H). Ethyl (E)-2-cyano-3-(2-furyl)-2-propenoate [30] (Table 1 , entry 5). Melting point 89-91 °C, 1H NMR (601 MHz, DMSO) δ 8.22 (s, 1H), 8.14 (s, 1H), 7.53 (d, J = 3.6 Hz, 1H), 6.88 (dd, J = 3.6, 1.7 Hz, 1H), 4.29 (q, J = 7.1 Hz, 2H), 1.29 (t, J = 7.1 Hz, 3H). 2-(4-hydroxyphenylmethylene)malononitrile [6] (Table 1 , entry 6). Melting point 183-186 °C, 1H NMR (601 MHz, CDCl3) δ 7.85 (d, J = 8.8 Hz, 2H), 7.63 (s, 1H), 6.94 (d, J = 8.8 Hz, 2H), 4.75 (s, 1H). 2-(4-nitrophenylmethylene)malononitrile [31] (Table 1, entry 7). Melting point 159-160 °C, 1H NMR (601 MHz, CDCl3) δ 8.37 (d, J = 8.8 Hz, 2H), 8.06 (d, J = 8.7 Hz, 2H), 7.86 (s, 1H). 2-(4-chlorophenylmethylene)malononitrile [27] (Table 1, entry 8). Melting point 158-159 °C, 1H NMR (601 MHz, CDCl3) δ 7.83 (d, J = 8.6 Hz, 2H), 7.72 (s, 1H), 7.50 (d, J = 8.6 Hz, 2H). 2-(4-methylphenylmethylene)malononitrile [29] (Table 1, entry 9). Melting point 122-125 °C, 1H NMR (601 MHz, CDCl3) δ 7.79 (d, J = 8.2 Hz, 2H), 7.70 (s, 1H), 7.31 (d, J = 8.1 Hz, 2H), 2.43 (s, 3H). 2-(2-furylmethylene)malononitrile [30] (Table 1, entry 10). Melting point 68-70 °C, 1H NMR (601 MHz, CDCl3) δ 7.79 (d, J = 1.6 Hz, 1H), 7.50 (s, 1H), 7.34 (d, J = 2.9 Hz, 1H), 6.70 (dd, J = 3.7, 1.7 Hz, 1H). References [S1] Y.X. Xu, H. Bai, G.W. Lu, C. Li, G.Q. Shi, J. Am. Chem. Soc. 130 (2008) 5856–5857. [S2] V. Brahmkhatri and A . Patel, Ind. Eng. Chem. Res. 50 (2011) 6620–6628.