pola27796-sup-0001-suppinfo01
advertisement

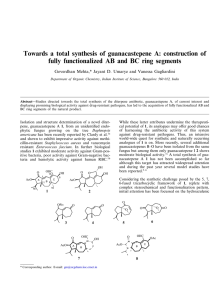
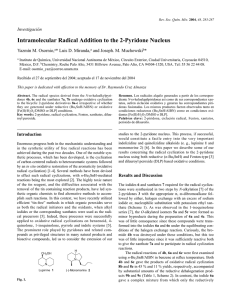
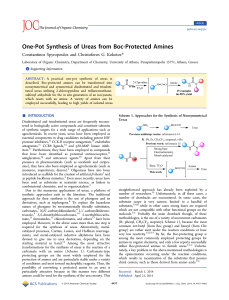
SUPPORTING INFORMATION A ROUTE TOWARD MULTI-FUNCTIONAL POLYURETHANES USING TRIPLE CLICK REACTIONS Erhan Demirel, Hakan Durmaz, Gurkan Hizal, Umit Tunca* Department of Chemistry, Istanbul Technical University, Maslak, Istanbul 34469, Turkey *correspondence to U. Tunca (E-mail: tuncau@itu.edu.tr) EXPERIMENTAL Synthesis of perfluorophenyl 2,2,5-trimethyl-1,3-dioxane-5-carboxylate 2,3,4,5,6-pentafluorophenol (3.00 g, 16.3 mmol, 1 equiv) was dissolved in 100 mL of CH2Cl2 and 2,2,5-trimethyl-1,3-dioxane-5-carboxylic acid (3.40 g, 19.6 mmol, 1.2 equiv), and DMAP (0.398 g, 3.26 mmol, 0.2 equiv) were added to the reaction mixture in that order. After stirring for 5 min at room temperature, DCC (4.04 g, 19.6 mmol, 1.2 equiv) dissolved in 20 mL of CH2Cl2 was added to the solution, followed by stirring overnight at room temperature. Finally urea byproduct was filtered and the reaction mixture was extracted with CH2Cl2 two times, and combined organic phases were dried with Na2SO4. Solvent was evaporated and the remaining product was purified by column chromatography over silica gel eluting with hexane/ethyl acetate (9/1) to give white solid (Yield = 4.58 g; 83%). 1H NMR (CDCl3, δ) 4.32 (d, J=11.9 Hz, 2H, CCH2O), 3.79 (d, J=11.8 Hz, 2H, CCH2O), 1.48 (s, 3H, CCH3) 1.43 (s, 3H, CCH3), 1.34 (s, 3H, C=OC(CH2O)2CH3); 19F NMR (CDCl3, δ) -152.95 (d, J=17.6 Hz, 2F, o-F), -157.81 (t, J=21.7 Hz, 1F, p-F), -162.32 (t, J=20.0 Hz, 2F, m-F). 1 Synthesis of perfluorophenyl 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate (perfluorophenyl diol) (3) Perfluorophenyl 2,2,5-trimethyl-1,3-dioxane-5-carboxylate (4.58 g, 13.5 mmol) was dissolved in a mixture of 40 mL of THF and 40 mL of 1 M HCl. The solution was stirred for 2 h at room temperature and the precipitated product was filtered off. The reaction mixture was concentrated and extracted with CH2Cl2. The combined organic phase was dried with Na2SO4 and the solvent was evaporated under reduced pressure to give perfluorophenyl diol (white solid) (Yield=3.96 g, 98%). 1H NMR (CDCl3, δ) 4.07 (d, J=11.0 Hz, 2H, CH2OH), 3.88 (d, J=10.9 Hz, 2H, CH2OH), 1.31 (s, 3H, C=OC(CH2O)2CH3);19F NMR (CDCl3, δ) -152.74 (d, J=17.3 Hz, 2F, o-F), -157.47 (t, J=21.6 Hz, 1F, p-F), -162.09 (t, J=21.7 Hz, 2F, m-F). Figure S1. 1 H NMR spectra of 2,2,5-trimethyl-1,3-dioxane-5-carboxylic acid (a); perfluorophenyl 2,2,5-trimethyl-1,3-dioxane-5-carboxylate (b); perfluorophenyl diol (3) in CDCl3 (500 MHz). 2 Figure S2. 19 F NMR spectra of perfluorophenyl 2,2,5-trimethyl-1,3-dioxane-5-carboxylate (a); perfluorophenyl diol (3) (b) in CDCl3 (470.4 MHz). Figure S3. 19F NMR spectrum of PU-(anthracene-co-alkyne-co-perfluorophenyl) in CDCl3 (470.4 MHz). 3 Figure S4. 19F NMR spectrum of PU-(anthracene-co-benzyltriazole-co-perfluorophenyl) in CDCl3 (470.4 MHz). Figure S5. IR spectra of PU-(anthracene-co-alkyne-co-perfluorophenyl) (before CuAAC) PU(anthracene-co-benzyltriazole-co-perfluorophenyl) (after CuAAC). 4 Figure S6. 19F NMR spectrum of PU-(anthracene-co-benzyltriazole-co-octylamine) in CDCl3 (470.4 MHz). Figure S7. UV spectra of PU-(anthracene-co-benzyltriazole-co-octylamine) (C0 = 0.172 g/L) (at 0 h) (before Diels-Alder reaction) and PU-(hydoxyl-co-benzyltriazole-co-octylamine) (after Diels-Alder reaction) (at 48 h) in CH2Cl2. 5