Gas Notes
advertisement

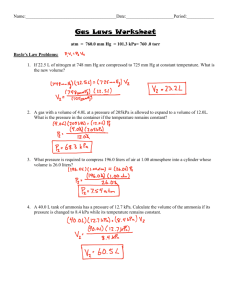
Honors Chemistry I Gas Laws Notes Why gases can be studied collectively while liquids and solids cannot. Differences of volume, shape, and mixtures of gases vs. liquids & solids Difference between the term “gas” vs. “vapor” Pressure - concept of pressure - view from a physics perspective F = ma F = mg - consider the mass of the column of air that is pressing down on you - density of air is 1.29 g/liter - units / conversions 1 atmosphere = 14.7 psi = 760.0 mm Hg = 29.92 in. Hg = 101.3kPa = 760 torr = 76.0 cm Hg - work of Evangelista Torricelli (1608 - 1647) o - standard conditions - 0 C and 1 atm. (STP) Sample problem: Convert 11.8 pounds per square inch of pressure to the following units: = _____________ atm. = _____________ psi = _____________ kPa = ___________ inches Hg = = _____________ torr _____________ mm. Hg - recording of temperature must be done on the Kelvin scale - relationships of the kinetic energies of the gases are based on the absolute scale - means of measuring pressure - mercury barometer - significance of Torricelli vacuum - water barometer Sample problem: If the atmosphere pressure was reading 75.3 cm.Hg in a regular mercury barometer, what would the height of a water barometer be, in feet? - manometer -confined gas vs. atmospheric pressure Sample problem: A manometer is hooked up to a confined gas. If the atmospheric pressure is 30.25 inches Hg, and the column of mercury on the side attached to the cylinder of gas is 2.25 inches higher than the side exposed to the atmosphere, what is the pressure of the confined gas, in atmospheres? -2Gas Laws - Boyle's: V x P = k; then V1P1 = V2P2 (inverse relationship) (Robert Boyle - 1627-1691) Sample problem: A 4.00-liter sample of gas, originally at 782 torr, has the pressured decreased to 98.8 kPa. What is the volume of the gas at the reduced pressure? - Charles': V/T = k; then V1/T1 = V2/T2 (direct relationship) (Jacques Charles -1746-1823) Sample problem: A 275-milliliter sample of gas, originally at 125oC, has the temperature doubled to 250oC. What is the volume of the gas at the increased temperature? - discuss work of William Thompson (Lord Kelvin) - Gay-Lussac's: Law of Combining Volumes N2 + 3 H2 ------> 2 NH3 1 liter + 3 liters = 2 liters - Amonton's: P/T = k; then P1/T1 = P2/T2 (direct relationship) - Avogadro's: V/n = k; then V1/n1 = V2/n2 (direct relationship) - Combined Gas Law Equation if P x V = k and V/T = k and P/T = k then P1V1 ------ = T1 P2V2 -----T2 Sample problem: A 2.00-liter sample of gas, originally at STP conditions, has the pressure increased to 25.8 psi and the temperature decreased to 233 K. What is the volume of the gas at the new conditions? -3Ideal Gas Equation: PV = nRT - show how “R” is determined . . R = 0.0821 liter atm / mole K - derived forms - molecular weight determination MW = gRT / PV - density determination: since D = M/V; then D = (P)(MW) / RT Sample problems: 1. What is the pressure (in kPa) of a chlorine gas if the temperature is 75.9oC on 525 ml of the gas? The mass of this sample of gas is 14.4 grams. 2. What is the molecular mass of an unknown gas if 4.00 grams, when placed into a 2.00-liter container, exerts a pressure of 25.5 psi at a temperature of 425 K? 3. What is the density of carbon monoxide gas at STP conditions? 4. What is the density of this same gas if the pressure is 2.45 atmopsheres and the temperature is 225 K? Dalton's Laws of Partial pressure - two forms 1. Ptotal = PA + PB + PC + ... (esp. useful in a 2-gas system where a gas is collected over water) 2. PA = (Ptotal)(XA) where X = mole fraction of “A” = moles of A / total # of moles Sample problems: 1. Air is basically 77.8% nitrogen by weight and 22.2% oxygen. What is the partial pressure (in mm Hg) exerted by the oxygen in air if the atmospheric pressure is 766 torr? 2. A sample of hydrogen gas is collected over water. If the aqueous vapor pressure at 25oC is 19.8 mm Hg, what is the pressure of dry hydrogen at the same temperature? The atmospheric pressure at lab conditions is 75.5 cm Hg. -4Kinetic Molecular Theory (5 points): 1. All gas molecules are considered to be “point” masses. 2. The average kinetic energy of any gas is a function of its absolute temperature. At any given temperature, the gas molecules of all gases have the same average kinetic energy. 3. Gas particles move in a rapid, random, straight-line motion. o ex. nitrogen gas @ 25 C moves at a rate of about 1150 mph (515 m/sec) 4. The volume of the gas molecules are negligible compared to the volume in which they are contained. 5. Attractive and repulsive forces between gas molecules are negligible. Energy can be transferred between molecules that collide, but the collisions are perfectly elastic. 19 (@ room temp, the number of collisions is about 10 per ml per second! Graham's Law of Effusion - difference between diffusion vs. effusion - derivation of rate formula - comparing rate to MW - comparing time to MW - comparing density to MW Sample Problems: 1. What is the molecular mass of an unknown gas if it is known to effuse at a rate of 3.45 liters per minute while oxygen gas effuses through the same chamber at a rate of 7.78 liters per minute? 2. What is the rate of effusion for carbon monoxide if helium gas effuses at a rate of 750 ml per second? 3. An unknown gas effuses X moles in 24.5 minutes. If it takes fluorine gas only 19.95 minutes to effuse X moles, what is the molecular mass of the unknown gas? -5Deviations from ideal behavior - two points of deviation 1. low temperature & its effect 2. high pressure & its effect - van der Waals equation Stoichiometry involving gases Sample problem: 1. In the complete combustion of methane gas, how many liters of carbon dioxide gas are produced if 1.25 moles of methane are used initially? Assume STP conditions. 2. A student reacts 2.67 moles of nitrogen gas with 6.50 grams of hydrogen gas. The reaction occurs at 225 K and 22.3 psi. How many liters of ammonia gas should be produced? Acidic properties of nonmetallic oxide gases