Honors Chemistry Chapter 13 Practice Quiz How many liters of CO2
advertisement

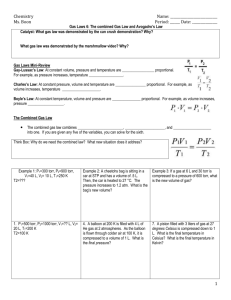
Honors Chemistry Chapter 13 Practice Quiz 1. How many liters of CO2 will the combustion of two liters of acetylene (C2H2) produce? (4L assuming STP) 2. What are the partial pressures of 3 moles He, 2 moles O2, 5 moles N2, and 4 moles CH4 of the total pressure is 760 mm Hg? (163% He, 109 % O2, 271% N2 and 217% CH4 assuming 273 K) 3. If we have a mixture of two gasses, O2 and N2, how fast on average are the O2 molecules moving if the N2 molecules are moving at 500 m/s? (468 m/s) 4. What pressure of 3 L O2 at 293 K will contain the same number of molecules as 2 L N2 at 55 C and 1.5 ATM? (0.89 L) 5. What is the volume of a gas at 700 Torr which was measured to be 1000 cm3 at 760 Torr? (1086 Torr) For Question 6, assume STP. 6. We have two balloons. Balloon A has a volume of 22.4 L, and balloon B has a volume of 11.2 L. a. If balloon A has one mole of He, how many moles of N2 are in balloon B? b. Which balloon has the fastest moving molecules? c. Which balloon has the highest kinetic energy? d. Which balloon has the highest pressure? e. What is the density of each of the balloons as described in Q A? (0.179 g/L He and 1.21 G/L N2) f. What is the new density of the balloons if the pressure drops to 750 mm and the temperature increases to 298 K? (0.161 g/L He and 1.13 g/L N2) 7. Express .5 ATM as: a. Bar b. Torr c. Mm Hg d. kPa e. psi 8. Sketch each graph: P V T P T V V 9. Define absolute zero 10. What is temperature? a. kinetic energy of molecules b. velocity of molecules c. mass of molecules 11. If the volume of a gas is doubled when the pressure is halved, what happens to its temperature? a. stays the same b. increases c. decreases 12. Which of the following is the smallest pressure? a. 101 kPa b. 78 Torr c. 900 Kelvin d. 25 ATM e. 4 mm Hg 13. A gas is heated from -137 C to -37 C. What happens to its volume if the pressure is held the same? a. Increases 3.7 times b. doubles d. halves e. decreases 3.7 times 14. Define STP 15. When sodium carbonate is added to hydrochloric acid, carbon dioxide, water and sodium chloride are formed. How many liters of CO2 are formed when 200 g of sodium carbonate are reacted in excess HCl? (42.3 L) Moles