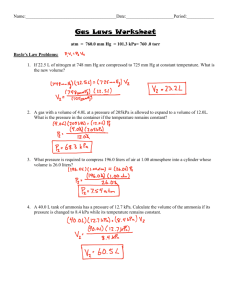
11_19_20 Gas Law Review
advertisement

Unit 5: Gases – Review Session 11.19 & 20 Ms. Boon Chemistry Objectives: •I can use the gas laws to solve gas law problems and explain the physical properties of gases. Agenda • • • • Catalyst Liquid Nitrogen Demo Gas Law Stations Jeopardy Catalyst • Can you use the gas laws to open a bag of hot cheeto’s without using your hands? (Hint: think about changes in pressure.) HW: Complete all Gas Law Unit Worksheets. Liquid Nitrogen Demo • Background: Liquid Nitrogen is very cold. • Focus Question: What gas law is demonstrated in the video? Explain. • http://www.youtube.com/watch?v=ZvrJgGhnm Jo&feature=player_embedded Gas Law Stations Review • Station 1 (Door): Gases Memory Game – work with a partner. Instructions are at the table. • Station 2: Gas Law Poster Practice – Get up and walk around. Complete 10 gas law problems from the posters. • Station 3 (lion): Gas Law Problem Sort – work with a partner. Sort the problems onto the game board according to the gas law you would use to solve. • Station 4: Reading/Independent Study – Choose an article to read or continue working on your gas laws notes packets. Exit Slip 1. 2 Liters of a gas at STP is expanded to 4 Liters and 273 K. What is the final pressure? 2. A balloon at 546 K, 4L, and 2 atm is cooled down to STP. Find the final volume. 3. What is standard temperature and pressure expressed in Kelvin and atmospheres? 4. What is temperature? 5. What is the relationship between volume and pressure? What is the name of the gas law that represents this relationship? Jeopardy! • • • • jeopardylabs.com/play/gas-laws-review5 Each table is a team. Write your answer on the white board. If your table gets the question incorrect, your table loses points. • If your table gets the question incorrect, you can choose the table to pass the question to. • Review video: http://watchknowlearn.org/Video.aspx?VideoID=3 0087&CategoryID=4848