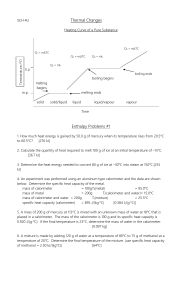
CHAPTER 4 – CHEMICAL REACTIONS
advertisement

CHAPTER 6 – THERMOCHEMISTRY THERMOCHEMISTRY: study of interconversion between thermal energy (heat) and chemical energy. Law of Conservation of Energy: Energy cannot be created or destroyed, it can only be converted from one form to another. 1st Law of Thermodynamics: The total amount of energy of the universe is constant. A chemical system exchanges energy with its surroundings through heat. System – the reaction or process you’re interested in. i.e. CH4 + 2 O2 CO2 + H2O + heat Surroundings Surroundings – everything else System Exothermic Process: heat produced in process (heat released to surroundings) Surroundings Endothermic Process: heat put into process System UNITS OF ENERGY Calorie (C): nutritional energy unit Calorie = 1000 calories (kilocalorie) calorie (c): amount of energy required to raise temperature of 1.00 g of water by 1 oC Joules (J): amount of energy required to move a 1 kg mass a distance of 1 meter (kg * m2/s2) *1 calorie = 4.184 joules (J) A baked potato has a nutritional value of 120.0 Calories. How many kilojoules (kJ) is this? ENTHALPY (H): heat added or lost in a chemical reaction at constant pressure. Change in Enthalpy (H): change in heat between reactants and products. H = Hfinal – Hinitial H = Hproducts – Hreactants Endothermic Reactions H is positive (+) heat added to system (system absorbs heat from surroundings) example: cold packs What is H for the following endothermic reaction? i.e. H2O(l) + 286 kJ H2(g) + ½ O2(g) H = ________ k J Exothermic Reactions H is negative (-) heat leaves system (surroundings absorb heat from system) i.e. hot packs What is H for the following reaction? Is it endo or exothermic? i.e. H2O(g) H2O(l) + 44 kJ H = _______kJ Is sweat evaporating from your skin “the cooling effect” an endothermic or exothermic process? What is H? SPECIFIC HEAT (Cs) Amount of heat energy required to raise the temperature of one gram of a substance 1oC (units: J/goC). Sensitivity of a substance to heat. (easier to change temp of substance lower specific heat, i.e. copper) Substance Specific Heat Water Copper 4.184 J/goC 0.385 J/goC Why is Santa Monica cooler than Woodland Hills? HEAT CAPACITY (C) – Amount of heat required to raise a substance’s temperature by 1 oC. (units: J/oC) Dependent on mass of substance. Calculating the heat (q) that goes into a pure substance q = m X Cs X T q = amount of heat (J) m = mass of substance (g) Cs (specific heat) = J/g oC T = TempFinal – TempInitial Calculate the amount of heat transferred to a 2.00 gram copper sample where the temperature rose from 293K to 617 K (specific heat of Cu = 0.385 J/g K)? q = m X Cs X T What is the specific heat of silicon if it takes 192 J to raise the temperature of 45.0 g of Si by 6.0 oC? Calorimetry: Measures heat evolved in a chemical reaction, where heat exchanged between reaction (system) and surroundings is measured by the change in temp. of surroundings. Amount of heat gained by calorimeter equals amount of heat released by system. qcalorimeter = - qrxn Bomb Calorimeter: A device to measure the heat released during a combustion reaction. Heat transferred to water and calorimeter. Calculate the amount of heat (kJ/g) produced and the Hrxn for the combustion reaction of 1.00 g of octane in a bomb calorimeter. The calorimeter contains 1.20 kg of water, has a heat capacity of 837 J/K and the temperature rises from 25.00 oC to 33.20 oC during combustion. Energy to heat water: q H2O = mass H2O X Cs H2O X T H2O Energy to heat calorimeter: q calorimeter = C calorimeter X T H2O Total energy produced: q surroundings = q H2O + q calorimeter q surroundings = -q system Hrxn: q system / gram Calculate the amount of heat (kJ/mol) transferred to the surroundings from 500.0 mg of benzoic acid, C7H6O2, that undergoes combustion in a bomb calorimeter, when the temperature rises from 20.00oC to 23.01oC. The calorimeter contains 950.0 grams of water and has a heat capacity of 420.0 J/oC. Energy to heat water: q H2O = massH2O X Cs H2O X TH2O Energy to heat calorimeter: q calorimeter = Ccalorimeter X TH2O Total energy transferred: q surroundings = q H2O + q calorimeter q surroundings = -q system Energy transferred per mole of benzoic acid: Coffee Cup Calorimeter: A device to measure the heat released by a substance or from a chemical reaction. Determine the specific heat of a 55.0 g metal sample that was heated to 99.8 oC and placed into a coffee cup calorimeter. The calorimeter contained 225 mLs of water and temperature rose from 21.0 oC to 23.1 oC after the metal was added. (assume calorimeter did not absorb heat) Heat gained by calorimeter = heat lost by metal q H2O = -q metal Calculate the enthalpy change, Hrxn, kJ per mole of magnesium, for the reaction between 0.500 g of Mg and 100.0 mLs of a 1.00 M HCl solution in a coffee cup calorimeter. The temperature of the solution increases from 22.2 oC to 44.8 oC. (assume specific heat sol’n = 4.20 J/gK; density =1.0 g/mL) 2 HCl(aq) + Mg(s) Determine limiting reactant: H2(g) + MgCl2(aq) Hess’s Law: The change in enthalpy for a stepwise process is the sum of the enthalpy changes of the steps. Hrxn = H1 + H2 + …. Example: Calculate Hrxn (kJ) for the following combustion reaction. C6H6(l) + 15/2 O2 (g) 6 C(s) + 3 H2 (g) H2(g) + ½ O2 (g) C (s) + O2 (g) 3 H2O(l) + C6H6(l) H2O(l) CO2(g) 6 CO2(g) H1 = +49.0 kJ H2 = -285.8 kJ H3 = -393.5 kJ Standard Enthalpy Change (Ho): The change in enthalpy when reactants and products are in standard state. Standard State Conditions: P = 1 atm and T= 25 oC Standard Enthalpy of Formation (Hof): the energy associated (H) from the formation of one mole of a compound from its elements. Hof for pure compounds value obtained from data table Hof = 0 for pure elements H2(g) + ½ O2 (g) H2O(l) Hof = -285.8 kJ/mol Write an equation for the formation of MgCO3 from its elements in their standard states and include Hof. Engineer’s Shortcut Horxn = n Hof (products) – n Hof (reactants) Calculate Horxn for the following reaction: C6H6(l) + 15/2 O2 (g) Compound Hof (kJ/mol) C6H6 (l) CO2 (g) H2O (l) +49.0 -393.5 -285.8 3 H2O(l) + 6 CO2(g)