ACID – BASE TITRATION
advertisement

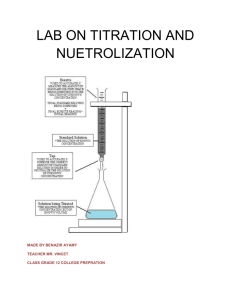
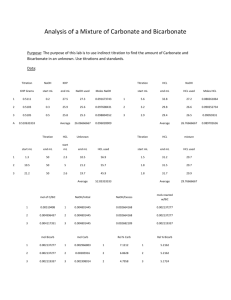
ALKALIMETRY (ACID-BASE TITRATION) Task 8.1. Purpose: Titration is a procedure for determining the concentration of the solution using another solution of known concentration, called standard solution or titrant. There are three types of titration: titrations involving a strong acid and a strong base; titrations involving a weak acid and a strong base; titrations involving a weak base and a strong acid. Titrations involving a weak acid and a weak base are complicated by the hydrolysis of both the cation and the anion of the salt formed and for this reason they are almost never carried out. The reaction between HCl, a strong acid, and NaOH, a strong base, can be presented by NaOH(aq) + HCl(aq) NaCl(aq) + H2O(l) or , in terms of the net ionic equation, H+ (aq) + OH- (aq) H2O(l) At the equivalentce point both the hydrogen ion and hydroxide ion concentrations are very small. Addition of a single drop of the base can cause a large increase in [OH -] and the solution pH. For monitoring pH during the course of a titration pehameter or indicatores are used. The purpose of experiment is determination of acid concentration (HCl) by adding NaOH solution of exactly known concentration by the acid-base titration method. Reagents 0.10M NaOH solution ( from a burette) HCl solution ( unknown concentration) indicator ( phenolphtaleine) Laboratory equipments 250 cm3 volumetric flask 50 cm3 pipette 250 cm3 Erlenmeyer flasks (3) 50 cm3 graduated cylinder 50 cm3 burette Experimentation: HCl solution of unknown concentration is distributed in the volumetric flask by the demonstrator. Add the distilled water up to the mark of 250cm3 and mix By means of the pipette, Erlenmeyer flasks remove the solution thoroughly. 50cm3 of acid solution to each of the three and dilute with distilled water to about 100 cm3. Remember that before removal of acid , the pipette must be washed with acid solution. Add 1-3 drops of phenolophtaleine into each of the Erlenmeyer flasks. Fill up the burette with 0.10 M of NaOH solution up to the zero mark ( bottom menisque). Add 0.10 M NaOH solution drop-wise from the burette while stirring slowly till the solution in the flask becomes pink . When the solution becomes pink you must finish the titration process and read the total volume of 0.10 M NaOH solution. It is an equivalent point of acid-base titration. The experiment must be repeated three times ( 3 flask). Therefore the burette must be filled up with 0.10M NaOH solution up to the zero mark. Repeat the procedure for flasks 2 and 3. Calculate the average volume of 0.10M NaOH solution from the three measurements Calculate the molar concentration of HCl acid. Calculation The concentration of H+ (HCl) in solution is found as follows: C B · VB C A · VA = CB · VB CA = CA= acid concentration [mol/dm ] VA VA = acid volume [cm3] CB = base concentration [mol/dm3] VB= base volume 3 [cm3] Results Sample NaOH No conc. [mol/dm3] 1 2 3 0.10 0.10 0.10 NaOH volume [cm3] HCl conc. [mol/dm3] HCl volume [cm3] NaOH average volume [cm3] HCl conc. [mol/dm3