Chemistry 11 Periodic Trends Practice Sheet Name: ______ Given
advertisement
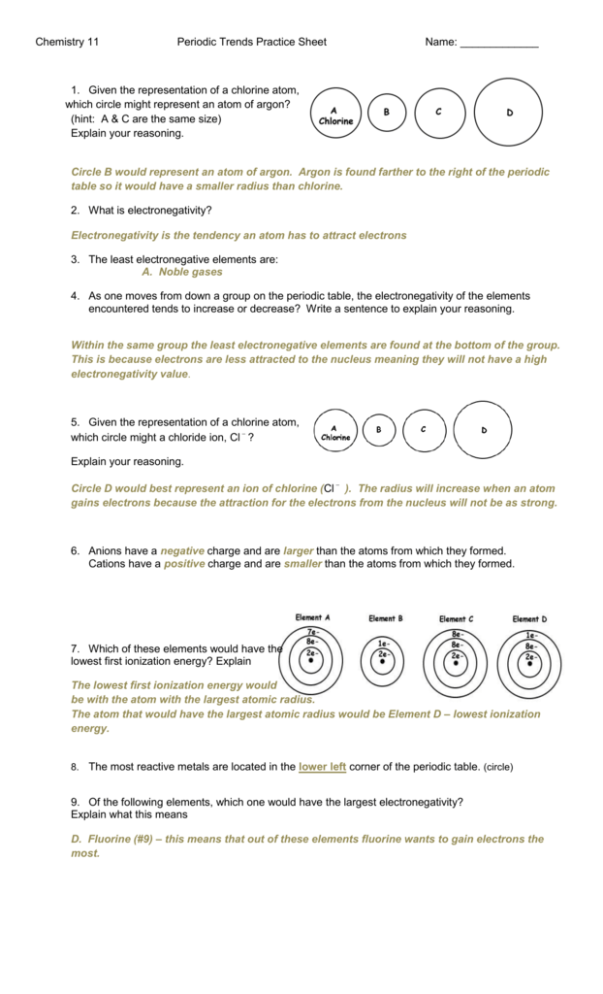
Chemistry 11 Periodic Trends Practice Sheet Name: _____________ 1. Given the representation of a chlorine atom, which circle might represent an atom of argon? (hint: A & C are the same size) Explain your reasoning. Circle B would represent an atom of argon. Argon is found farther to the right of the periodic table so it would have a smaller radius than chlorine. 2. What is electronegativity? Electronegativity is the tendency an atom has to attract electrons 3. The least electronegative elements are: A. Noble gases 4. As one moves from down a group on the periodic table, the electronegativity of the elements encountered tends to increase or decrease? Write a sentence to explain your reasoning. Within the same group the least electronegative elements are found at the bottom of the group. This is because electrons are less attracted to the nucleus meaning they will not have a high electronegativity value. 5. Given the representation of a chlorine atom, which circle might a chloride ion, Cl ? Explain your reasoning. Circle D would best represent an ion of chlorine (Cl ). The radius will increase when an atom gains electrons because the attraction for the electrons from the nucleus will not be as strong. 6. Anions have a negative charge and are larger than the atoms from which they formed. Cations have a positive charge and are smaller than the atoms from which they formed. 7. Which of these elements would have the lowest first ionization energy? Explain The lowest first ionization energy would be with the atom with the largest atomic radius. The atom that would have the largest atomic radius would be Element D – lowest ionization energy. 8. The most reactive metals are located in the lower left corner of the periodic table. (circle) 9. Of the following elements, which one would have the largest electronegativity? Explain what this means D. Fluorine (#9) – this means that out of these elements fluorine wants to gain electrons the most. 10. Metalloids are elements that show: C. both metallic and nonmetallic properties 11. What does it mean when we talk about 2nd and 3rd ionization energies? 2nd ionization energy is the amount of energy require to remove the 2nd electron. 3rd ionization energy is the amount of energy require to remove the 3rd electron. 12. Low ionization energies are most characteristic of: A. Metals 13. The energy required to remove an electron from an atom is known as: D. ionization energy 14. The measure of the attraction that an atom has for electrons involved in chemical bonds is known as: B. electronegativity 15. The elements with the largest atomic radii are found in the A. lower left-hand corner of the periodic table 16. The elements with the smallest atomic radii are found in the B. upper right-hand corner of the periodic table Given the representation of a chlorine atom, which circle might represent an atom of sulphur? D 17. Using the above diagram of a chlorine atom, which circle might represent an atom of fluorine? B 18. Using the above diagram of a chlorine atom, which circle might represent an atom of bromine? D 19. Which of the following elements is a metalloid? C. Germanium (#32) 20. Generally speaking, the group of elements with the highest first ionization energy is the noble gases. And the lowest ionization energy is alkali metals. Explain why. The group that contains the atoms with the smallest atomic radii will have electrons that are strongly attracted to the nucleus. This means that the electrons will be held tightly and require a lot of ionization energy. The noble gases do not want to lose any electrons (they are already stable) so they will have high IE. The group that contains atoms with the largest atomic radii will have electrons that are not as attracted to the nucleus. This means that electrons will not be held tightly and have low 1st ionization energy. This group is the alkali metal group. 21. Generally speaking, the group of elements with the highest electronegativity is halogens. And the lowest electronegativity is the noble gases. Explain why. The halogens are the group that wants to gain electrons the most – they want to have a full valence shell (think p6). The noble gases do not want to gain any electrons; this group of atoms is already stable. This means they will have the lowest electronegativity values. 22. The halogen group wants to gain one electron, but the alkali metal group wants to lose one electron. Explain why this is. To be stable the atoms are trying to obtain p6 in their last level of electron configuration. When alkali metals lose one electron they obtain this electron configuration and when halogens gain one electron they also obtain this electron configuration.