Chemistry Name: Unit 5 – Trends/Nomenclature Review Date
advertisement

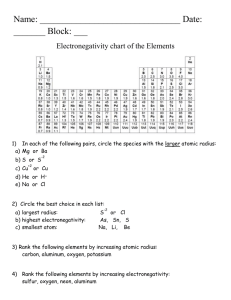
Chemistry Unit 5 – Trends/Nomenclature Review Name:_______________________ Date:__________________ Periodic Trends: Using the general trends; circle the correct answer: 1. Which has a larger atomic radius, K or Mn? 2. Which is the larger atom, W or Os? 3. Which atom is smaller, Cd or Y? 4. Which atom has lower ionization energy, Rb or Li? 5. Which atom has a higher electronegativity, N or As? 6. Which has a smaller atomic radius, I or Cl? 7. What atom would have the largest radius? _____ 8. What atom would have the lowest ionization energy? _____ 9. What atom would have the highest electronegativity? _____ 10. Non-metals generally have ____________ atomic radii than metals. 11. Which atom in period 3 would have the lowest electronegativity? 12. Which atom in group 7 would have the highest ionization energy? 13. Which alkaline earth metal has the largest ionic radius? 14. All atoms want to have 8 valence electrons in their highest principal energy level. This is called the ________ rule. 15. Which noble gas has 2 valence electrons? ____ Why does it not follow the octet rule like the rest of the noble gases? 16. Which alkali metal (not including H) has the highest ionization energy? 17. Which d-block metal has the highest electronegativity? 18. ______________ are larger than their neutral atoms. Are they usually metals or nonmetals? 19. ______________ are smaller than their neutral atoms. Are they usually metals or nonmetals? 20. The _____________ metals are the most reactive of all the metals. 21. The ______________ are the most reactive non-metals. 22. The _________________ are unreactive because they have 8 valence electrons. 23. Fluorine is 1 electron away from obtaining ____________________ configuration. 24. What element is in group 6, period 5? _____ 25. Write the symbol of an element that is not a good conductor of heat and electricity. _____ 26. Write the symbol of the element that is in period 6, group 10. _____ 27. Metals _________ electrons to form positively charged ions called ________. 28. Non-metals _________ electrons to form negatively charged ions called ________. 29. Why does the F1- ion easily form and the N3- ion rarely form? 30. Use your nomenclature worksheets to study for that portion of the test The answer keys on on my website).