JKKENNON PERIODIC TRENDS STUDY GUIDE “THE HOW, THE
advertisement
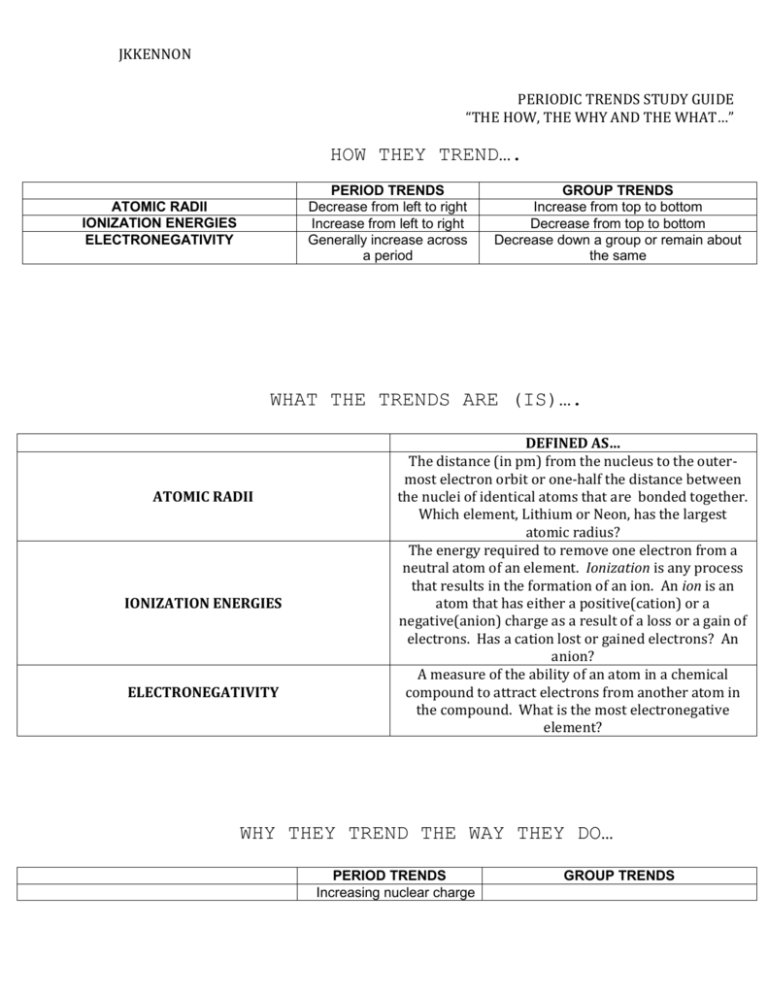
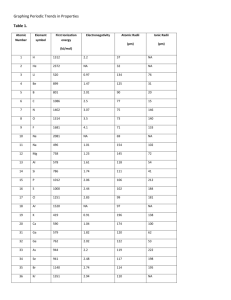
JKKENNON PERIODIC TRENDS STUDY GUIDE “THE HOW, THE WHY AND THE WHAT…” HOW THEY TREND…. PERIOD TRENDS Decrease from left to right Increase from left to right Generally increase across a period ATOMIC RADII IONIZATION ENERGIES ELECTRONEGATIVITY GROUP TRENDS Increase from top to bottom Decrease from top to bottom Decrease down a group or remain about the same WHAT THE TRENDS ARE (IS)…. ATOMIC RADII IONIZATION ENERGIES ELECTRONEGATIVITY DEFINED AS… The distance (in pm) from the nucleus to the outermost electron orbit or one-half the distance between the nuclei of identical atoms that are bonded together. Which element, Lithium or Neon, has the largest atomic radius? The energy required to remove one electron from a neutral atom of an element. Ionization is any process that results in the formation of an ion. An ion is an atom that has either a positive(cation) or a negative(anion) charge as a result of a loss or a gain of electrons. Has a cation lost or gained electrons? An anion? A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. What is the most electronegative element? WHY THEY TREND THE WAY THEY DO… PERIOD TRENDS Increasing nuclear charge GROUP TRENDS JKKENNON ATOMIC RADII IONIZATION ENERGIES ELECTRONEGATIVITY or more protons are added to the atom causing the nuclear pull on the electrons to increase.. Because as the atom becomes increasingly more like a noble gas electron configuration (or achieves noble gas stability), it requires more energy to ionize a neutral atom of that element. See below More sublevels are added to the atom, (1s, 2s, 3s, etc…). Because of shielding and because the electrons are in higher energy levels and thus are further from the positive nuclear pull. Therefore, it requires less energy to ionize a neutral atom as one trends from top to bottom down a group. Shielding occurs when more electrons lie between the nucleus and the electrons in the highest occupied energy levels. See below JKKENNON JKKENNON JKKENNON ATOMIC RADII JKKENNON