Help the rainbow fish find his colors!
advertisement

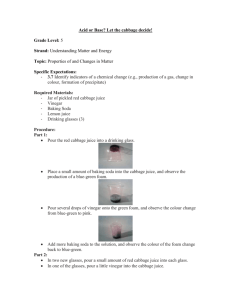
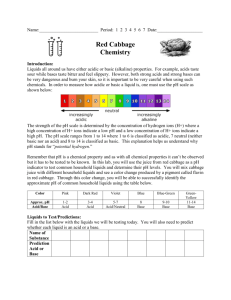
Teacher Instructions Help the Rainbow fish find his colors! Grades K-4 Ana Shapiro January 11, 2010 The objective of this lab is to define physical and chemical changes, acids and bases and to observe the different chemicals involved in black inks, washable markers and colored candies. Any of a chemicals properties that are observed during a chemical change Chemical Property Any of a chemical’s properties that are observed during a chemical change. Examples: flammability, acidity, how basic a chemical is Intrinsic property an inherent property like color or density, a property that does not depend upon how much of an object is present Extrinsic property a nonessential or inherent property of an object that may depend on how much of an object is present such as mass or height Acid any compound that gives on hydrogen ions when dissolved in a solution, it must have a pH lower than 7 Base a substance that can accept hydrogen ions, it must have a pH higher than 7 pH a substance’s ability to give off or accept hydrogen ions, the scale is 114 neutral- having a pH of 7 Materials: Cabbage juice ice cubes (can be made in Dixie cups) Clear glasses Vinegar Baking soda Water Cabbage juice Straws Coffee filters 1 Black pen Black permanent marker Black washable marker Colored washable markers Skittles Procedure Give each group a glass of vinegar, a glass of water and a glass of water mixed with about a tablespoon of baking soda. Also give each group three cabbage juice ice cubes, have them drop one in each glass and see what happens. Discuss what happens. Before putting the frozen cabbage juice in the solutions discuss which solution is acidic, which is neutral and which is basic. Then discuss what the cabbage juice test tells us. Now pass around a glass of cabbage juice and give each student a straw. Tell them to blow bubbles into the cup for a moment and then pass it on. After it has been passed around hold the cup up to another cup of cabbage juice that has not had bubbles blown into it. The first cup is purple now instead of blue. This means that blowing bubbles into the glass has made it slightly acidic because the CO2 from our breath is dissolved in the solution (mention that many gasses can be dissolved in liquids). The reaction of CO2 and H2O produces carbonic acid. Prior to the chromatography section of the lab, cut the coffee filters into strips. Give each group three initially and have them draw a black dot on each using the washable marker on one, the black pen on one and the permanent marker on one. Then place the strips into a beaker with a small amount of water at the bottom of it. Have the class draw what happens. Repeat the procedure with colored markers and then with skittles. (Pigment can be taken from the skittles by rinsing them with water and then using a finger to dab a bit onto the strip. Have the students draw what happens. Discuss what is happening and how different black inks may involve different chemicals, some that are colors other than black. Time Requirements This lab should take about an hour. If running out of time, you can cut down on the chromatographies or assign different chromatography tests to different groups. References The Usborne Big Book of Experiments by Alastair Smith Championship Science Fair Projects by Sudipta Bardhan-Quallen 2 Name:_____________________ Help the Rainbow fish find his colors! Part One: Use deep ocean blue and clear liquids to make pink and blue green. You have three clear solutions, vinegar, water and baking soda and water. Draw lines to show which is an acid, which is a base and which is neutral. Vinegar Base Baking soda solution Neutral Water Acid Draw what happens in each solution after you add the dark blue ice cube (this is frozen cabbage juice). Water Vinegar Baking Soda Solution What happens when cabbage juice interacts with an acid? What happens when cabbage juice interacts with a base? What happens when cabbage juice interacts with a neutral solution? What does cabbage juice tell us about a solution? 3 Part 2: Find the colors in black! Place a black dot about one inch above the end of each strip of coffee filter paper using the black pen, the black washable marker and the black permanent marker. Pour a very small amount of water into the beaker then carefully place each strip in, with the dot side facing the water (but try to make sure the dots don’t actually touch the water). What does the water do as it touches the filter paper? After the water passes the black dot what happens? Does the same thing happen for each black dot? After 5 minutes draw what each strip looks like Part 3: What colors can we find in other colors? Use the washable colored markers to dot filter paper and place the strips in the water to see what happens. Also you can see what colors we can find from the coloring on skittles by running water over the skittle, and then using your finger to dab a bit of color onto a filter strip. Do any of the colors surprise you? 4 Draw the strips and be sure to label which color the initial dot was for each strip. 5