Honors Biology NAME: Ch. 2- Chemistry of Life DATE: PERIOD
advertisement

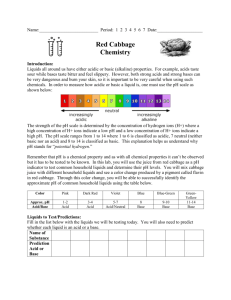
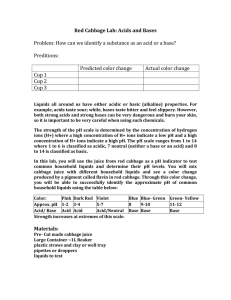
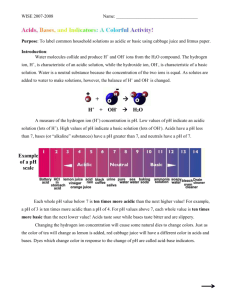
Honors Biology Ch. 2- Chemistry of Life NAME: ____________________________________________ DATE: _______________ PERIOD: _____________ ________ 27 pH Lab Review of Acids and Bases: Complete the sentences with the help of your notes and textbook. A water molecule (H2O) can split apart to form a(n) __________________________________ ion and a(n) __________________________________ ion. The __________________________________ is a scale that measures the concentration of hydrogen ions in a solution. The scale ranges from 0 to 14. Pure water has a pH of __________________________________. A(n) __________________________________ is any compound that forms H+ ion in solution. __________________________________ have a pH level below 7. A(n) __________________________________ is a compound that forms OH- ions in solution. __________________________________ have pH values above 7. __________________________________ are weak acids or bases that react with strong acids or bases to prevent sudden changes in pH. They are very important in maintaining homeostasis in living things. Introduction: Liquids all around us have either acidic or basic (alkaline) properties. For example, acids taste sour while, bases taste bitter and feel slippery. However, both strong acids and strong bases can be very dangerous and burn your skin, so it is important to be very careful when using such chemicals. In order to measure how acidic or basic a liquid is, one must use the pH scale as illustrated below: The easiest way to determine if a substance is acidic or a basic is to use an indicator. Indicators are organic molecules that change color in an acid or a base. In this lab, you will use the juice from red cabbage as a pH indicator to test common household liquids and determine their pH levels. You will mix cabbage juice with different household liquids and see a color change produced by a pigment called flavin in red cabbage. Through this color change, you will be able to successfully identify the approximate pH of common household liquids using the table below: Materials: - Cabbage juice - 7 plastic cups Liquids to test: - Bleach - Lemon juice - Vinegar - Apple juice - Color-coded pH scale - Graduated cylinder - Baking soda solution - Baby shampoo - Ammonia Safety Rules: YOU MUST WEAR GLOVES & GOGGLES FOR THE DURATION OF THE EXPERIMENT. Never taste chemicals (or other substances) used for a lab experiment. Do not mix chemicals unless specifically directed. Clean up spills immediately. If any substance gets into your eyes or in a cut on your skin, notify your teacher and follow his/her directions. Wash your hands before and after an experiment. Clean up your lab area and materials after an experiment and return materials to their proper location. Procedure: NB: Follow the instructions carefully. Do not skip steps. 1. Label each cup with each of the liquids. 2. Using a graduated cylinder, pour 50 mL of each test liquid into its respective cup. 3. CAREFULLY pour 25 mL of cabbage juice into the first test cup. In the data table, write down the color change IMMEDIATELY after you pour the cabbage juice. Use the pH scale below to find what pH this color change corresponds to. 4. Repeat Step 3 for every test cup, writing down the color change after every new test. 5. Identify whether each test liquid is an acid, a base, or neutral. TEST LIQUID Bleach Lemon Juice Vinegar Apple juice Baking Soda Baby shampoo Ammonia COLOR OF INDICATOR APPROXIMATE pH ACID/BASE/NEUTRAL? Data Analysis Create a graph using the pH data. Label one axis “pH” and the other axis “Test Liquids.” (2 pts) Conclusion Answer each question related to the experiment. 1. In this experiment what was the independent variable? (1 pt) 2. In this experiment what was the dependent variable? (1 pt) 3. In general, how did the acids affect the color of the indicator? How did the bases affect the color of the indicator? (2 pts) 4. Compare the concentrations of H+ and OH- ions in acids. Compare the concentrations of H+ and OH- ions in bases. (2 pts) Clean-up checklist: Please do not use an excessive amount of paper towels. While the water is running, pour all liquids into the sink. Throw away all cups and gloves. Rinse out Return goggles. Wipe down any liquids on the bench or floor. Teacher’s initials: _________________ = 3 pts