Compounds and mixtures
advertisement
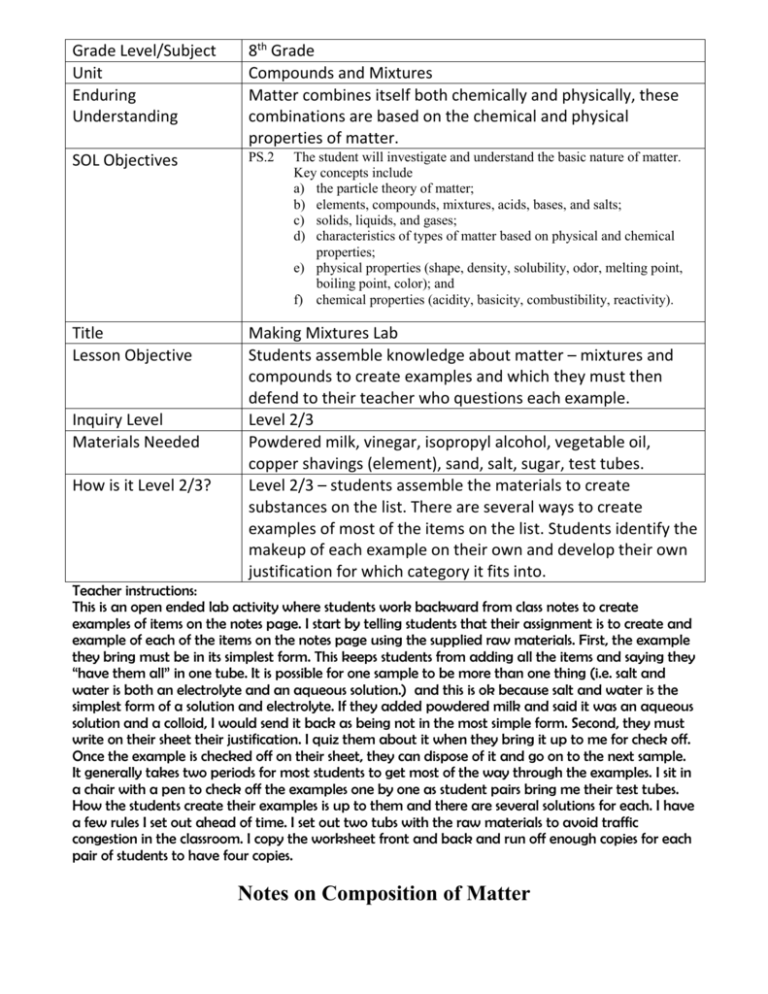
Grade Level/Subject Unit Enduring Understanding 8th Grade Compounds and Mixtures Matter combines itself both chemically and physically, these combinations are based on the chemical and physical properties of matter. SOL Objectives PS.2 Title Lesson Objective Making Mixtures Lab Students assemble knowledge about matter – mixtures and compounds to create examples and which they must then defend to their teacher who questions each example. Level 2/3 Powdered milk, vinegar, isopropyl alcohol, vegetable oil, copper shavings (element), sand, salt, sugar, test tubes. Level 2/3 – students assemble the materials to create substances on the list. There are several ways to create examples of most of the items on the list. Students identify the makeup of each example on their own and develop their own justification for which category it fits into. Inquiry Level Materials Needed How is it Level 2/3? The student will investigate and understand the basic nature of matter. Key concepts include a) the particle theory of matter; b) elements, compounds, mixtures, acids, bases, and salts; c) solids, liquids, and gases; d) characteristics of types of matter based on physical and chemical properties; e) physical properties (shape, density, solubility, odor, melting point, boiling point, color); and f) chemical properties (acidity, basicity, combustibility, reactivity). Teacher instructions: This is an open ended lab activity where students work backward from class notes to create examples of items on the notes page. I start by telling students that their assignment is to create and example of each of the items on the notes page using the supplied raw materials. First, the example they bring must be in its simplest form. This keeps students from adding all the items and saying they “have them all” in one tube. It is possible for one sample to be more than one thing (i.e. salt and water is both an electrolyte and an aqueous solution.) and this is ok because salt and water is the simplest form of a solution and electrolyte. If they added powdered milk and said it was an aqueous solution and a colloid, I would send it back as being not in the most simple form. Second, they must write on their sheet their justification. I quiz them about it when they bring it up to me for check off. Once the example is checked off on their sheet, they can dispose of it and go on to the next sample. It generally takes two periods for most students to get most of the way through the examples. I sit in a chair with a pen to check off the examples one by one as student pairs bring me their test tubes. How the students create their examples is up to them and there are several solutions for each. I have a few rules I set out ahead of time. I set out two tubs with the raw materials to avoid traffic congestion in the classroom. I copy the worksheet front and back and run off enough copies for each pair of students to have four copies. Notes on Composition of Matter Name Definition Examples Elements Substances that cannot be broken down into simpler substances. Elements can be found on the periodic table of the elements Copper, aluminum, lead, anything that can be found on the PTOE. Compound Two or more elements chemically combined Water, salt, sugar It is not two substances placed together in a test tube such as salt and water. Heterogeneous Mixture in which the substances are not mixed evenly throughout. Any substances that won’t evenly mix. Homogeneous Mixture in which the substances are mixed evenly throughout. Pure substances - Elements and compounds, solutions (salt water and sugar water. Not: Sandy water. Not milk - milk is actually not evenly mixed on the microscopic level. Solution Homogeneous mixture in which the particles are so small they cannot be seen with a microscope. Solutions remain permanently mixed. Salt water, sugar water. Not: Milk, sandy water Suspension: Heterogeneous mixture, in which the visible particles suspended in the fluid will eventually settle out when not mixed. Sandy water. Anything in the water that eventually settles down to the bottom. Colloid Heterogeneous mixture that never settles. Particles are large enough to scatter light, but not usually large enough to see. Milk Not: Sandy water, or other substances that will settle out. Immiscibles Liquids that will not dissolve in each other Oil and water. Two LIQUIDS that won’t mix. Some types of salad dressings are immiscible. Electrolyte Electrolytes will conduct electricity. They are created when ions are dissolved in water Salt water. Salt is an ion. Not: anything else. Tincture Solution in which alcohol is a solvent. Salt or sugar and alcohol. Aqueous Solution in which water is the solvent. Anything dissolved in water. Insoluble Two substances that will not dissolve in each other. Sand and water. Copper and water. Or either in alcohol. Solids or liquids. Unsaturated The solvent is not full of solute. When more solute is added, it will dissolve. Adding just a little bit of salt to water will create an unsaturated solution Saturated The solvent is full of solute. If more solute is added it will not dissolve, it will sink to the bottom. Adding enough salt to the water that some of the salt settles on the bottom and never dissolves. Supersaturated This is a solution that is over saturated. When a solution is cooled, its ability to hold a solute is decreased. Therefore a super saturated solution is created when a saturated solution is cooled. Take water and add so much salt to it that the salt settles on the bottom, shake repeatedly. Place the solution in the refrigerator. When the solution is removed it will be cooled. Cooling it reduces its ability to hold the salt that it is already holding, so it becomes supersaturated. Not: Water, salt, sugar Other Examples ? Making Mixtures See if you can make any of the things listed to the left. Then explain in as much detail as possible why you believe that what’s in your test tube is what you say it is. You can check the book pages 517 - 527 or notes sheet handed out in class. True Solution Colloid Suspension Immiscible liquids Insoluble Supersaturated solution Saturated solution Electrolyte Tincture Aqueous solution Element Compound Homogeneous Heterogeneous What is it? _________________________ What is it made of? _____________________________________________________________ _____________________________________________________________________________ For what reasons, is it what you say it is? ______________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ What is it? _________________________ What is it made of? _____________________________________________________________ _____________________________________________________________________________ For what reasons, is it what you say it is? ______________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Name__________ Date___________