Lab Report Solubility Experiment
advertisement

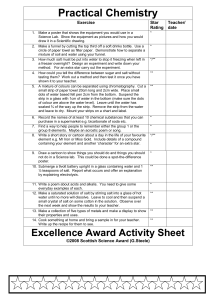
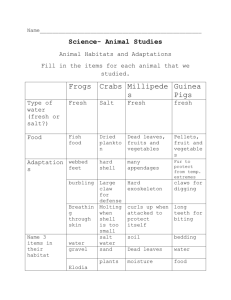
Name: ________________________ Date: ___________________ Lab Report – Grade 7 Science Pure Substances and Mixtures Name of Experiment: _________________________________________ Testable Question: Hypothesis: (Prediction) Equipment Used: 1. Test tubes 2. Stoppers 3. Measuring spoons 4. Cold water, room temperature water and hot water 5. Empson salts Procedure: Controlled variables: __________________________________________________ Independent variable: _________________ Dependent variable: _____________________ 1. Have one member of the group time for agitation to make sure the solution is agitated (shaken) for 1 minute each time. 2. Pour 5ml of salt in each of the water tubes and agitate for set time. 3. Record both your quantitative and qualitative observations in the chart each time more salt is added. 4. Repeat, carefully recording how much salt is added to each tube before it stops dissolving. 5. When the salt is no longer dissolving the solution has reached its saturation point. 6. Carefully measure the amount of saturated solution before you pour down the sink. Data and Observation: (Present your observations in a form that is easily understood. Record in tables with units of measurement and qualitative observations.) Amount of solute Cold Water: 5 g (1 teaspoon) Qualitative Observations 10 g (2 teaspoons) 15 g (3 teaspoons) Quantity of saturated solution at end of experiment: _________ Hot Water: Quantity of saturated solution at end of experiment: _________ Quantitative Observations Analysis and Evaluation: The amount of salt added up to the point of saturation can be used to calculate the concentration of salt in the solution. Calculate the concentration for the solutions you made at each temperature: Concentration of cold solution: Concentration of warm solution: Evaluate: Write a conclusion of your experiment and indicate whether or not your results support your hypothesis or prediction. Add a reflection: How did the experiment go? Could you have done things better? Other notes or questions: