Supplementary Methods (doc 66K)
advertisement

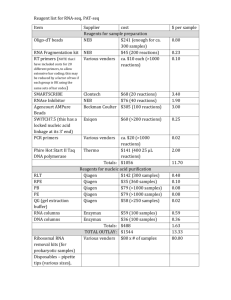
Supplementary Methods Immunohistochemistry Immunohistochemistry was performed using a panel of antibodies specified in supplementary table 1, according to the previously published techniques1. Detection was carried out on either a Ventana Benchmark automated immunostainer using UltraView detection, or a Dako Autostainer Plus using either Envision+ or Flex detection systems. Detailed protocols are available on request. Fluorescence-in-situ-hybridization Interphase fluorescence in-situ hybridization (FISH) studies were performed on 5 microns Formalin-Fixed-Paraffin-Embedded (FFPE) tumor sections using standard protocols2. For all cases, FISH was performed using the D7S522 (7q31)/CEP7 probe (Abbot Molecular, Illinois, U.S.A.). Some cases were also tested for loss of chromosome 7p copy number using 7p11.2/7q31 probe mix: (7p11.2, BAC clones RP11-56P1, RP11-371M7 in Spectrum Green, Empire Genomics)/ (7q31, Spectrum Orange, Empire Genomics). Hybridizations were performed according to standard protocols using a ThermoBrite denaturation/hybridization system (Abbot Molecular, Illinois, U.S.A.). Image acquisition and analysis were performed on the BioView Duet-3 fluorescent scanning system using 63X-oil objective and DAPI/FITC/Rhodamine single-band pass filters (Semrock, Rochester, NY). At least 100 interphase cells were analyzed for each patient. T-cell receptor gamma-chain gene rearrangement studies DNA from each case was extracted using the Qiagen QIAamp DNA FFPE Tissue Kit on a QIAcube robotic system, according to the instructions of the manufacturer. PCR was performed using consensus primers to the T-cell receptor variable and joining regions. The primers used interrogate primers all known Vγ family members and the Jg1/2, JP1/2, and JP joining segments. The products were analyzed by either acrylamide gel electrophoresis or by capillary electrophoresis on an ABI 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA). Mutational Analysis of STAT3 and STAT5B Targeted analysis for STAT3 mutations located between codons 614 through 661 and for STAT5B mutation located between codons 642 through 665 were performed using pyrosequencing on a PyroMark Q24 instrument (Qiagen, Alameda, CA). The pyrosequencing assay was designed to target all hotspot mutations identified in recent publications3-6 using PyroMark Assay Design v2.0 (Qiagen). PCR amplification primers and sequencing primers are as follows: STAT3 codons 640-665 assay (118 bp amplicon), STAT3-FW 5'- TAAGACCCAGATCCAGTCCG, STAT3-REV 5'-biotin-CCAGTGGAGACACCAGGATATTG, STAT3-S1 5'CCAGTCCGTGGAACC, STAT3-S2 5'-TGAAATCATCATGGGC; STAT3 codons 614-618 assay (102 bp amplicon), STAT3-FW2 5'-CGGGCCATCTTGAGCACTAA, STAT3-REV2 5'-biotin- GATGTCCTTCTCCACCCAAGTG, STAT3-S3 5'-GCTAAGATTCAGTGAAAGC; STAT5B codons 642-665 assay (122 bp amplicon), STAT5b-FW 5'-TTGATCTAGAGGAAAGAATGTTTTGG, STAT5B-REV5'biotin CCGATCAGGAAACACGTAGATAA, STAT5b-S 5'CTAGAGGAAAGAATGTTTTG. PCR reactions were conducted in a total volume of 25 ul containing genomic DNA template, 200 nM of each forward and reverse primers, and 12.5 ul 2x HotStarTaq Master Mix (Qiagen) with conditions at 95ºC 15 min, 40x (95ºC 20 sec, 60ºC 30 sec, 72ºC 20 sec), 72ºC 10 min, 15ºC hold. For the pyrosequencing reactions, 10 ul of PCR product was immobilized on streptavidin-coated Sepharose beads (GE Healthcare) and processed according to the manufacturer’s instructions. References 1. Garcia-Herrera A, Song JY, Chuang SS, Villamor N, Colomo L, Pittaluga S, et al. Nonhepatosplenic gammadelta T-cell lymphomas represent a spectrum of aggressive cytotoxic T-cell lymphomas with a mainly extranodal presentation. The American journal of surgical pathology 2011 Aug; 35(8): 1214-1225. 2. Pack SD, Zhuang Z. Fluorescence in situ hybridization : application in cancer research and clinical diagnostics. Methods in molecular medicine 2001; 50: 35-50. 3. Jerez A, Clemente MJ, Makishima H, Koskela H, Leblanc F, Peng Ng K, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood 2012 Oct 11; 120(15): 3048-3057. 4. Kontro M, Kuusanmaki H, Eldfors S, Burmeister T, Andersson EI, Bruserud O, et al. Novel activating STAT5B mutations as putative drivers of T-cell acute lymphoblastic leukemia. Leukemia 2014 Feb 27. 5. Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmaki H, Andersson EI, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. The New England journal of medicine 2012 May 17; 366(20): 1905-1913. 6. Rajala HL, Eldfors S, Kuusanmaki H, van Adrichem AJ, Olson T, Lagstrom S, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood 2013 May 30; 121(22): 4541-4550.








![Title Goes Here [BMCL TITLE] - HAL](http://s3.studylib.net/store/data/007683270_2-8e9977658a510f755c34103527b035c7-300x300.png)





