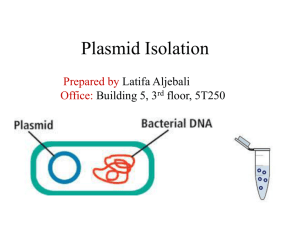
PROTOCOL FOR PLASMID ISOLATION

Experiment 2: Isolation of Plasmid DNA
Theory:
A plasmid is a small, circular, double-stranded DNA molecule that is distinct from a cell's chromosomal DNA. Plasmids naturally exist in bacterial cells, and they also occur in some eukaryotes. Often, the genes carried in plasmids provide bacteria with genetic advantages, such as antibiotic resistance. Plasmids have a wide range of lengths, from roughly one thousand DNA base pairs to hundreds of thousands of base pairs. When a bacterium divides, all of the plasmids contained within the cell are copied such that each daughter cell receives a copy of each plasmid. Bacteria can also transfer plasmids to one another through a process called conjugation.
Scientists have taken advantage of plasmids to use them as tools to clone, transfer, and manipulate genes. Plasmids that are used experimentally for these purposes are called vectors. Researchers can insert DNA fragments or genes into a plasmid vector, creating a so-called recombinant plasmid. This plasmid can be introduced into a bacterium by way of the process called transformation. Then, because bacteria divide rapidly, they can be used as factories to copy DNA fragments in large quantities.
Materials Required:
Microcentrfuge, 1.5mL eppendorfs, tubes stand, a set of pipettes (1000µL, 200µL and
100µL),
Chemicals Required:
Solution I (50 mM Tris , pH 7.4 -7.6 + 1 mM EDTA), freshly prepared solution II (20µL
10 N NaOH+200µL 5% SDS+780 µL distilled water), solution III (3 M potassium acetate, pH 4.8 – 5.0, 1 day old bacterial culture, chloroform, isopropanol, chilled 70% ethanol, distilled water.
Procedure:
1.
This protocol for plasmid isolation is mini-preparation method and is described by
Sambrook and Russel (2001).
2.
First of all take 1.5 mL of 1 day old Bacterial culture and shift it to 1.5 mL microcentrifuge tubes and centrifuge at 14,000 rpm for 30 seconds.
3.
After that remove the supernatant and resuspend the pellet in 100 µL ice cold solution-I and incubate it for 5 minutes at room temperature.
4.
Thereafter, add 200 µL of freshly prepared solution-II and mix it well by inverting tubes five times and then place it on ice for 5 minutes.
5.
Remove it from the ice and Add 150 µL of solution-III and again incubate on ice for 5 minutes.
6.
Then centrifuge it at 14,000 rpm for 5 minutes at 4°C and transfer the supernatant to a new tube.
7.
Now add 500µL chloroform in the supernatant, mix it well and again centrifuge it at
14,000 rpm for 2 minutes at 4°C.
8.
Precipitate plasmid DNA 600µL chilled isopropanol to the supernatant and again centrifuge at 14,000 rpm for 5 minutes at 4°C.
9.
After centrifugation, add 500 µL 70% chilled ethanol.
10.
Discard ethanol and air dry the pellet.
11.
Resuspend the pellet in 50 µL of distilled water and store it at -20°C.
Precautions:
1.
Use sterilized eppendorfs.
2.
Note the centrifugation time accurately.
3.
Follow the time duration for each solution strictly; otherwise results can be badly affected.
4.
Must use chilled solutions of isopropanol and 70 % ethanol.