Lab 5-2 Decomposition Reactions
advertisement

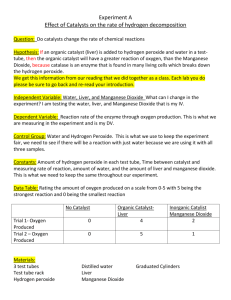
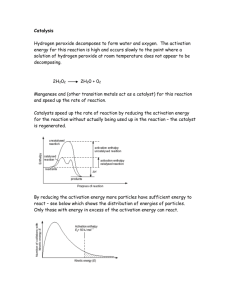
Lab 5-2 Decomposition Reactions (Covalent Bonds) Introduction: Decomposition reactions occur when a molecule breaks apart (decomposes) into two or more more stable parts. These parts may be simpler compounds or elements or both. Unlike replacement reactions, they do not partner with new elements. Some examples are: HgO ---> Hg + O2 CaCO3 ---> CaO + CO2 MgCl2 ---> Mg + Cl2 Na2CO3 ---> Na2O + CO2 FeS ---> Fe + S KClO3 ---> KCl + O2 Ba(ClO3)2 ---> BaCl2 + O2 In this lab we are going to start with hydrogen peroxide (H2O2) and it will decompose into two parts. We will be using manganese dioxide (MnO2) as a catalyst (something that speeds up a reaction but doesn’t get involved in the reaction). Sort of like when a girl walks into a classroom full of boys, the boys may start acting goofy, even though the girl doesn’t get involved. Materials: 50 mL graduated cylinder, test tube, beaker, hydrogen peroxide, manganese dioxide, matches, splint, goggles, apron. Purpose: To conduct, observe, & understand decomposition reactions & covalent bonding. To write the reaction equations and balance them. To understand the purpose of a catalyst. Hypothesis: The gas released by this reaction is _______________. Procedure: Decomposition Reaction – Hydrogen peroxide 1. Wear the lab apron and goggles at all times during this lab 2. Measure 14 mL of hydrogen peroxide and pour it into your test tube 3. Observe the hydrogen peroxide and record your observations in your data table. 4. Place a small amount (about 1/2 g or a spoon tip) of manganese dioxide into the cylinder. 5. Observe the reaction and record your observations in your data table. 6. Test for the presence of oxygen gas (light a splint & let it burn until the wood is glowing. Blow the flame out and then insert the glowing splint into the end of the test tube. Do not drop the splint.) Record your observations in your data table. 7. Dispose of the products by pouring it in the sink, with large amounts of water. 8. Wash and rinse your test tube thoroughly and place it upside down in your beaker to dry. Data Table: Design a data table to record your 3 sets of observations in this lab. Make sure it is clearly labeled. Questions: 1. What evidence(s) did you have of a chemical reaction taking place Use the diagram of the reactant hydrogen peroxide (H2O2) to answer some of the following questions: H O 2 e- O 2 e- H 2. Why do the hydrogen and oxygen atoms remain neutral instead of taking on a + or – charge? 3. Finish the reaction equation for this decomposition reaction started below. (Note: oxygen is the diatomic molecule O2 and you know what water is.) Reactants Products H2O2 (MnO2) 4. Draw diagrams for the products of this reaction (what does H2O2 break down into?). 5. Is this equation balanced (are the numbers of each kind of atom equal on each side of the reaction)? If not write the balanced equation. 6. What is the clue that tells you that the oxygen is released? 7. What is the black stuff in the test tube at the end of the reaction? 8. What is the liquid in the test tube at the end of the reaction? 9. What was the purpose of using the manganese dioxide (MnO2)? 10. What happened to the manganese dioxide in the reaction? Analysis & Reflection: Standard A&R plus include a diagram of the complete reaction (reactants & products).