Law of Definite Proportions Lab
advertisement

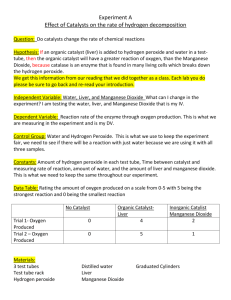
Law of Definite Proportions Lab Purpose: Students will be able to observe that compounds contain a definite number of atoms of each element. Therefore, students will observe that compounds can be made up of the same elements, but have totally different properties. Materials: 4 test tubes test tube rack 3% solution of hydrogen peroxide distilled water manganese dioxide wood splints matches bleach eye dropper (Warning: Manganese Dioxide and Hydrogen Peroxide are both poisonous. Keep them away from your face and wash your hands thoroughly after using these substances. Wear safety goggles throughout this lab.) Procedure: 1. Place two test tubes in a test tube rack. Into one test tube, pour approximately 5-mL of distilled water and into the other test tube pour approximately 5-mL of hydrogen peroxide. 2. Observe the physical properties of each compound and recorded your observations in the data table. (Found on the back side of this handout) 3. Put a small amount of manganese dioxide on the tip of a wood splint and add a little of this chemical to the test tube containing WATER. Record your observations on the data table. 4. Put a small amount of manganese dioxide on the tip of a wood splint and add a little of this chemical to the test tube containing HYDROGEN PEROXIDE. Record your observations on the data table. 5. If you saw evidence of a chemical reaction in either tube, prepare a glowing wood splint. Light the splint with a match so the wood begins to burn then blow out the flame but not so hard because you want to leave some of the wood GLOWING. Insert the glowing part of the splint into the test tube(s) that showed a reaction. Record your data in the data table. 6. Clean up both reaction set-ups. Flush contents of both test tubes down the drain with plenty of water. 7. Acquire a second set of test tubes and place in a test tube rack. Add approximately 1mL of bleach to both test tubes. WARNING: As you do these next few steps, be sure to keep your face and nose away from the open ends of the test tubes!!!! 8. Add a few drops of WATER to one of the test tubes. Record your observation in the data table. 9. To the second test tube, add a few drops of hydrogen peroxide. Record your observation in the data table. 10. Clean up both reaction set-ups. All liquids can go down the drain. 11. Wash your hands when you are finished with the lab Name ______________________________ Data Table: Reactions with Manganese Dioxide Compound Physical Properties of the liquid compound Evidence of a chemical reaction Distilled Water (H2O) Hydrogen Peroxide (H2O2) Data Table: Reactions with Bleach Reactants Observations: Bleach and Water (H2O) Bleach and Hydrogen Peroxide (H2O2) Questions: 1. Compare and contrast the molecular formulas of water and hydrogen peroxide. 2. Explain why water and hydrogen peroxide have different chemical properties. 3. Hydrogen gas burns. Oxygen gas supports burning of other materials. Which gas do you think was produced / were you testing for by doing the splint test? 4. How does The Law of Definite Proportions explain why water and hydrogen peroxide have different properties, although they consist of the same elements? 5. Why do you think hydrogen peroxide and bleach reacted as they did?