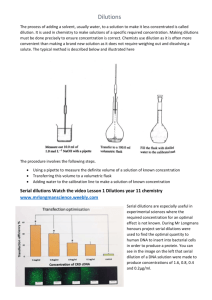
Dilutions: Practice
advertisement

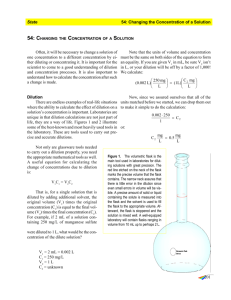
Dilution Chemistry In chemistry and biology we often need to make a dilute solution from a more concentrated solution in a process called dilution. Water is added to a concentrated solution to make a larger more dilute volume of solution. The concentration of the new solution can be expressed in Percent (%) Concentration or Molarity (M). The amount of solute doesn’t change when you make a dilution and thus the solute in the concentrated solution = amount of solute in the diluted solution. We can express this idea algebraically with the following equation. Using this equation we can determine the percent concentration or Molarity of any solution with a known. We use the letter ‘C’ or ‘%’ sign for the variable letter when determining a percent concentration of a solution. Percent concentration is mass/volume of solute. C1V1 = C2V2 where, C1 = initial concentration V1 = initial volume (ml) C2 = final concentration V2 = final volume (ml) We use the letter ‘M’ for the variable letter when determining Molarity of a solution. M1V1 = M2V2 where, M1 = initial concentration V1 = initial volume (ml) M2 = final concentration V2 = final volume (ml) Both equations are used and manipulated mathematically in the same way. Rev5Aug2014 Practice Calculations for Dilutions using Percent Concentration When working in the biology lab your instructor asks you to prepare the following concentrations of hydrogen peroxide (H2O2) for an upcoming lab experiment. The concentrated hydrogen peroxide is a 3% solution. Each dilution requires a final volume of 15 ml. Show all calculations for each dilution you will perform. Dilutions you need to prepare: 0.3% hydrogen peroxide 0.8% hydrogen peroxide 1.5% hydrogen peroxide Calculations: Practice Calculations for Dilutions using Molarity When working in the chemistry lab your instructor asks you to prepare the following dilute solutions from an initial concentrated stock solution of 0.01 M phenol red. Each dilution requires a final volume of 10 ml. Dilutions you need to prepare: 0.0001 M, 0.0003 M and 0.0005 M phenol red. Calculations: Rev5Aug2014