PreLab Review
advertisement

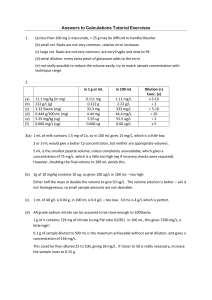
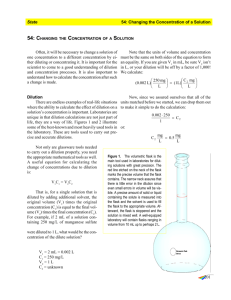
CHEM 1030L Fundamentals of Chemistry Laboratory Pre-lab Review - Experimental Design Week 1 Prepare for Laboratory 1. Review the following textbook section(s) 1.2 – Scientific Method: Thinking Like a Scientist 9.1 – Solutions 9.4 – Molarity (pages 298-299 only) 9.5 – Dilution of Solutions (use of formula: C1V1 = C2V2) 2. Review the following documents posted online. a. Pre-lab Assignment b. Lab Procedure 3. Be able to work the following textbook problems. Scientific Method: 1.7, 1.9, 1.33 Solutions: 9.1 Dilution: 9.55 4. Be able to summarize the purpose of the experiment. Two or three sentences summarizing what you will do, what you expect to learn, and what chemical or scientific principles you expect to understand better. Imagine what you would tell your roommate over a breakfast of Pop-Tarts about what you will be doing in lab that day. 5. Know and be able to do the following. a. Define the following terms experiment hypothesis scientific method theory solution solvent solute molarity dilution biofuel b. Identify steps in the scientific method (textbook section P.2). c. Calculate molarity of a solution after it has been diluted. d. The two types of mechanical or physical pretreatment that will be attempted. e. The names and formulas of the three reagents used for chemical pretreatment. 6. Example questions a. Write the name or formula of the following compounds in the corresponding blank. HCl ____________________________________ sulfuric acid ____________________________________ KOH ____________________________________ b. Write the definition of dilution. c. Write the definition of molarity. d. Write the definition of solute. e. Briefly describe the scientific method. f. Draw a diagram that shows the steps of the scientific method and how they relate to each other. g. Right the equation used to calculate the concentration of a diluted solution. h. 15 mL of 6 M H2SO4 are added to a beaker. Enough distilled water is added to bring the total volume to 100 mL. Demonstrate how to calculate the final concentration of H 2SO4 in the diluted solution. i. Three drops of 2 M HCl are added to a test tube. 15 drops of water are added. Demonstrate how to calculate the final concentration of hydrochloric acid in the diluted solution. j. Name the three reagents used for chemical pretreatment. k. Name the two types of mechanical or physical pretreatment that will be used.