HIBBING COMMUNITY COLLEGE
advertisement

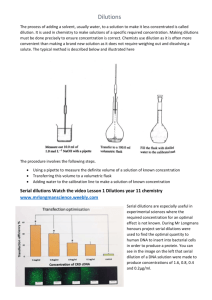
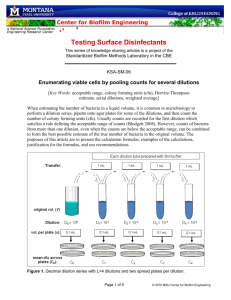
HIBBING COMMUNITY COLLEGE COURSE OUTLINE COURSE TITLE & NUMBER: MLT 1420: Laboratory Techniques CREDITS: 4 (Lecture Hours 34 / Lab Hours 68 per Semester) PREREQUISITES: None CATALOG DESCRIPTION: Laboratory Techniques is an introductory course in basic medical laboratory techniques. The equipment and techniques to be studied include laboratory glassware, pipetting, balances, centrifuges, solution chemistry, titration, spectrophotometry, and basic laboratory mathematics. This is the first in a series of clinical chemistry courses designed to teach fundamental concepts in clinical laboratory procedures. OUTLINE OF MAJOR CONTENT AREAS: I. Laboratory glassware II. Dilutions A. Calculations involving one dilution B. Volume dilutions C. Concentration dilutions D. Serial dilutions III. Molarity and Normality IV. Solution Chemistry A. Weight per unit weight (w/w) B. Weight per unit volume (w/v) C. Volume per unit volume (v/v) V. Conversion from one expression of concentration to another VI. Colorimetry A. Beer’s law B. Relationship between absorbance and transmittance C. Linear and semilog graphs D. Standard curves COURSE GOALS/OBJECTIVES/OUTCOMES: The student will 1. match various types of laboratory glassware with its appropriate use. 2. define the following terms; sample volume, diluent volume, total volume, dilution factor. 3. describe the procedure for making a series of tube dilutions. 4. determine the concentration of any tube in a serial dilution. Hibbing Community College, a technical & community college an equal opportunity educator & employer 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. calculate final concentration when performing serial dilutions. compare and contrast dilutions and titers. calculate the amount of one solution needed to make a solution of a lesser concentration. describe the difference between molar and normal solutions and be able to calculate how to prepare solutions of given volume and normality or molarity. convert molar solution concentrations to normal solution concentrations. make a percent solution. use Beer’s law to calculate concentration of unknowns. convert between absorbance and transmittance values. describe the current use of graphs and standard curves in the clinical laboratory. calculate the concentration of a substance using a standard curve. obtain the concentrations of unknown by single standards and multiple standards. calculate the mean, median, and mode of a set of variables. calculate the standard deviation and coefficient of variation of a set of variables. define the following terms: shift, trend. apply prior knowledge to problem solving. recognize abnormal or unusual test results. recognize unacceptable quality control results. apply knowledge of established procedures to verify test results Lab Competencies The student will 1. demonstrate how to read the meniscus in volumetric flasks, pipettes, and graduated cylinders. 2. prepare solutions and dilutions as described in procedures and manufacturer’s instructions. 1. demonstrate an understanding of calculations using dilutions. 2. perform serial dilution procedures. 3. calculate the mean, standard deviation, and coefficient of variation for a sample of test results 4. calculate the upper and lower limits of acceptability for quality-control test results. 5. demonstrate safe and professional work habits. HCC COMPETENCIES MET: Working Productively & Cooperatively, Communicating Clearly & Effectively. Hibbing Community College, a technical & community college an equal opportunity educator & employer STUDENT CONTRIBUTIONS: Students are expected to attend all lectures and labs, complete assignments on time, and spend necessary study time to pass all exams. METHODS FOR EVALUATING STUDENT LEARNING: Performance appraisals, assignment completion, lab reports, unit tests and a final exam are the methods used to evaluate student learning. SPECIAL INFORMATION: (SPECIAL FEES, DIRECTIVES ON HAZARDOUS MATERIALS, TEXTBOOK USED, ETC.) Students are expected to purchase lab coats and a calculator. Powder-free gloves are supplied. 11/16/04 Date Approved Hibbing Community College, a technical & community college an equal opportunity educator & employer