electronegativity HW
advertisement

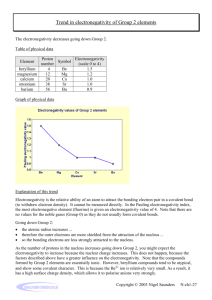
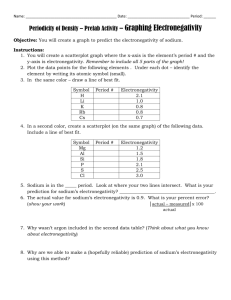
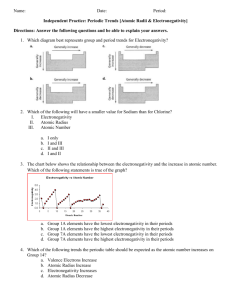
Last Name: First Name: Period # Unit 3: Chemical Bonding Electronegativity Homework! February 24, 2010 1. Rank the following elements by increasing electronegativity: carbon, aluminum, siliver, zinc. 2. Rank the following elements by decreasing electronegativity: sulfur, oxygen, boron, fluorine. 3. Why does fluorine have a higher electronegativity than iodine? 4. Rank the following elements in decreasing electronegativity: polonium, sulfur, oxygen, tellurium. 5. Why does boron have a lower electronegativity than fluorine? 6. Rank the following elements in decreasing electronegativity: antimony, neon, selenium, chlorine. 7. Why does electronegativity increase when atomic size decreases? 8. What element do you think has the highest electronegativity? Why? 9. What element do you think has the smallest atomic radius? Why? 10. Tyron argues that an element that electronegativity cannot affect atomic radius because they are opposites on the periodic table. Explain why they actually ARE related.