Study Guide for Chapter 4 Quiz: The Periodic Table Name: Identify
advertisement

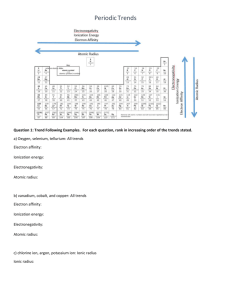
Study Guide for Chapter 4 Quiz: The Periodic Table I. Name:____________________ Identify families a. To which family does each of the following elements belong? _______________ Bromine ________________ Radium _______________ Rubidium ________________ Radon _______________ Astatine ________________ Einsteinium _______________ Argon ________________ Carbon _______________ Iron ________________ Arsenic _______________ Europium ________________ Lead II. Define trends a. Define each of the following terms: Ionization energy: Electron affinity: Electronegativity: Atomic radius: III. Compare elements based on trends. Fill in the chart with the words “Increases” or “decreases” Trend Ionization Energy Atomic Radius Electronegativity Electron Affinity Across a period (left to right) Down a family (top to bottom) IV. Put elements in order based on trends 1. ATOMIC RADIUS 3. IONIZATION ENERGY For each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. For each of the following sets of atoms, rank them from lowest to highest ionization energy. a. Mg, Si, S a. Li, C, F b. Mg, Ca, Ba b. Li, Na, K c. F, Cl, Br c. Ge, P, O d. Ba, Cu, Ne d. C, N, Al e. Si, P, He e. Al, Cl, Ga 4. ELECTRONEGATIVITY 2. IONIC RADIUS For each of the following sets of atoms, rank them from lowest to highest electronegativity. For each of the following sets of ions, rank them from smallest to largest ionic radius. a. Mg2+, Si4-, S2- a. Li, C, N b. Mg2+, Ca2+, Ba2+ b. C, O, Ne c. F-, Cl-, Br- c. Si, P, O d. Ba2+, Cu2+, Zn2+ d. K, Mg, P e. Si4-, P3-, O2- e. S, F, He V. Fill in the T-chart to compare and contrast Mendeleev and Moseley’s contributions to the development of the periodic table Mendeleev Both Moseley VI. Explain why the organization of the periodic table makes it useful. VII. Explain shielding and nuclear charge. Shielding: Nuclear Charge: VIII. Circle the correct choice in parenthesis to complete the definitions of periods and groups. Periods are (columns/rows) and their elements have the same number of (valence electrons/energy levels). Groups or families are (columns/rows) and their elements have the same number of (valence electrons/energy levels). IX. Identify metals, non-metals, semi-metals Label each element as a metal, non-metal, or semi-metal: __________________ Zinc ___________________ Germanium __________________ Nitrogen ___________________ Thallium __________________ Strontium ___________________ Osmium __________________ Neon ___________________ Chlorine __________________ Uranium ___________________ Silicon X. Properties of metals and non-metals a. Label the property as a metal or non-metal __________________ Ductile __________________ Does not conduct electricity __________________ Does not conduct heat __________________ Malleable __________________ Brittle __________________ Low melting point __________________ High boiling point __________________ Shiny