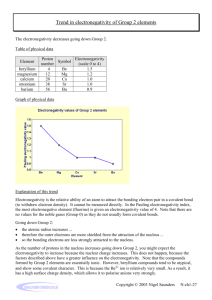
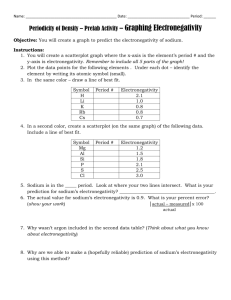
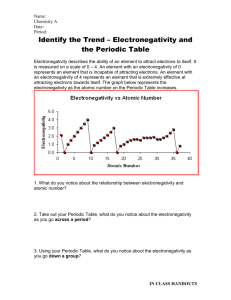
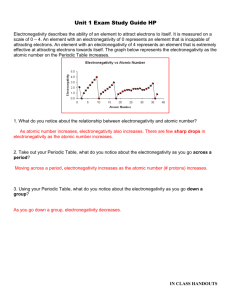
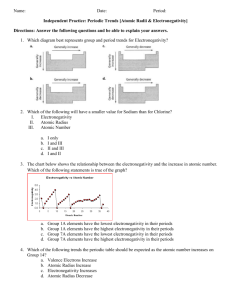
Which element in each pair has a higher electronegativity value?
advertisement

Electronegativity • Electronegativity is the ability of an atom in a compound to attract electrons. • Electronegativity values tend to decrease from top to bottom and increase from left to right. • Which element in each pair has a higher electronegativity value? • Mg or Ne • Mg • Which element in each pair has a higher electronegativity value? • Cl or F • F • Which element in each pair has a higher electronegativity value? • C or N • N • Which element in each pair has a higher electronegativity value? • As or Ca • Ca • Cs has one of the lowest electronegativity values. Valence (outer) electrons may be transferred from one atom to another. Group Valence elctrons Resulting charge 1A Lose 1 1+ 2A Lose 2 2+ 3A Lose 3 3+ 5A Gain3 3- 6A Gain 2 2- 7A Gain 1 1- • What charge would Na likely have in a compound? _______ • 1+ • What charge would Mg likely have in a compound? _______ • 2+ • What charge would Al likely have in a compound? _______ • 3+ • What charge would O likely have in a compound? _______ • 2• What charge would F likely have in a compound? _______ • 1-