EMISSION SPECTROSCOPY
advertisement

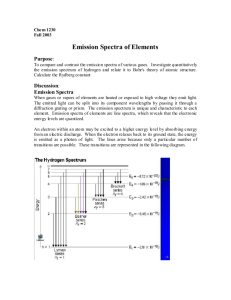
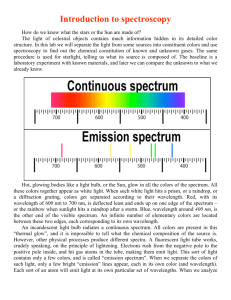
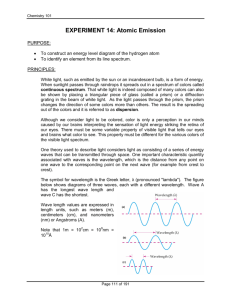
Topic: Emission Spectroscopy Lab Lesson Objectives: Students will be able to 1. use a spectroscope to examine continuous spectra from light sources and the bright-line spectra of some gaseous elements (3.4.10.A, 3.1.10.C, 3.1.12.C, 3.1.12.D, 3.2.10.B, 3.2.12.B). 2. calculate the energies that correspond to the wavelengths of bright-line spectra (3.4.10.A, 3.1.12.A, 3.1.10.B, 3.1.12.B, 3.1.10.D, 3.1.12.E). Materials: Lab Handouts Gas Tubes Containing the following: Spectroscopes (24) Hydrogen Argon Colored Pencils (6 packs) Helium Krypton Graph Paper Neon Xenon Gas Tube Power Supply (6) Procedures: Do a pre-lab discussion about the purpose, objectives, and safety concerns. Briefly go through a demonstration as to how to use the spectroscopes. Evaluation: Labs are evaluations of student understanding of content material. EMISSION SPECTROSCOPY Purpose: The purpose of this laboratory exercise is to observe the light produced by certain elements and calculate the amount of energy produced from the wavelength of this light. Discussion: All atoms give off electromagnetic radiation when their gases or ions are energized by heating or by a high voltage electric discharge. If the light emitted by a gas is passed through a spectroscope, a pattern of narrow lines of light is produced. Each element produces its own distinct pattern that differs from the pattern of every other element. The particular pattern of frequencies of light emitted by an atom is referred to as its emission spectrum, or bright-line spectra. The emission spectrum of an element can be used as a means of identification. Objectives: In this lab, you will... 1. use a spectroscope to examine bright-line spectra of several gaseous elements. 2. calculate the frequencies and energies that correspond to the wavelengths of bright-line spectra. Materials: Spectroscope Colored Pencils Graph Paper Gas Tube Power Supply Gas Tubes Containing the following: Hydrogen Argon Helium Krypton Neon Xenon Safety: The gas tube power supply carries a high voltage. Do not touch the glass tubes. Procedure: 1. Your instructor will load the glass spectrum tubes into the power source. 2. Using the spectroscope, examine the spectrum emitted by the gas in the glass tube. 3. Record your observations by drawing the spectrum using the colored pencils provided. Remember that spectra include spectral lines, so only draw vertical lines. For example… Hydrogen 400 nm 500 nm 600 nm 700 nm (draw vertical spectral lines here) 4. For hydrogen and helium, record the colors and wavelengths for the brightest spectral lines. Hydrogen should have three and helium should have seven. Be sure to record the wavelengths in nanometers to the nearest nanometer. Data: 1. Create a data table for all six element spectra. 2. Create a second data table for hydrogen and helium’s wavelengths (in nm and m), frequencies (calculated below) and energies (calculated below). Analysis: Show work for one example of calculation 1, 2 and 3. 1. In order for us to calculate frequency, wavelength must be in meters. Convert all wavelength values to meters. 1 m = 109 nm 2. Using the mathematical relationship between wavelength (in meters) and frequency, calculate the frequency for each spectral line. c = 299792458 m/s 3. Using the mathematical relationship between energy and frequency, calculate the amount of energy emitted for each spectral line. h = 6.62606876 x 10-34 J·s 4. Analyze the relationship between wavelength (in meters) and corresponding energy (in Data Table 2) by graphing the two variables on a piece of graph paper. Use the ten wavelength and energy values for both hydrogen and helium. Label your graph as Graph 1, and remember to provide a descriptive title. Remember the requirements for graphing outlined by your instructor. 5. Analyze the relationship between frequency and corresponding energy (in Data Table 2) by graphing the two variables on a second piece of graph paper. Use the ten frequency and energy values for both hydrogen and helium. Label your plot as Graph 2, and remember to provide a descriptive title. Error Analysis: 1. Calculate the percent error between the experimental energy values and the theoretical energy values for hydrogen. Use the given theoretical wavelengths and frequencies to match up the experimental energy values and the theoretical energy values. Data Table 3: Theoretical energies for the hydrogen line emission spectrum. Emission Line Color Blue-violet Blue-green Red Wavelength (nm) 434.037 486.1327 656.279 Frequency f (Hz) 6.90707 x 1014 6.166886 x 1014 4.56806 x 1014 Energy E (J) 4.57668 x 10-19 4.086225 x 10-19 3.02683 x 10-19 2. Interpret the percent errors you calculated in terms of errors that occurred in your lab experiment. Conclusions: 1. Why do atoms of gases larger than helium have more spectral lines? 2. Why do the colors of the spectral lines go from mostly bright reds, oranges and yellows for H, He, and Ne to dark greens, blues, and purples for Ar, Kr, and Xe? 3. Compare the results of your graphs to each other, relating their relationships to their mathematical relationships shown below. Explain whether the two variables are directly or inversely proportional. c = f, E = hf 4. How might the study of emission spectra be used in studying stars? 5. List and explain one other application of emission spectroscopy.