Spectral Lines Lab
advertisement

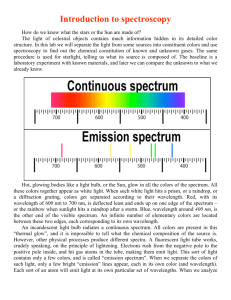
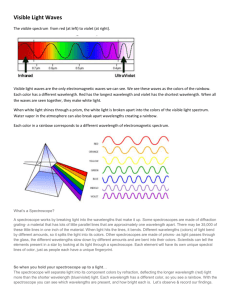
Physics/Honors Physics Spectral Lines Lab Name _______________________ Date _____________________ 1. We will use the voltage source to illuminate several spectrum tubes. Point the spectroscope at the spectrum tube and align the slit with the source, moving it until you see several sharp lines near the right side of the spectroscope 2. Draw the bright line spectral lines made by hydrogen, helium, nitrogen, neon, argon, and xenon below. Match these lines up with the numbers you see inside the spectroscope. If you are not using colored pencils, you will want to label the colors of the lines. Hydrogen 4 5 6 7 4 Nitrogen 5 6 7 4 Neon 5 6 7 4 Argon 5 6 7 4 4 Xenon 5 5 6 6 7 7 5 6 7 Helium 4 Questions 1. What is the job of a spectroscope? 2. View the fluorescent lights in the room through the spectroscope. Describe how the light you see is different than what you observed when you viewed the light from the gases? 3. Which particle in an atom gives off the light energy that produces spectral lines you observed? 4. Draw the bright line spectral lines you would expect to observe if you saw a mixture of hydrogen and helium through a spectroscope. Hydrogen and Helium 4 5 6 5. List the elements that you believe are in the unknown. (6 points) 1. _______________ 2. _______________ 3. _______________ 7