Western Blot
advertisement

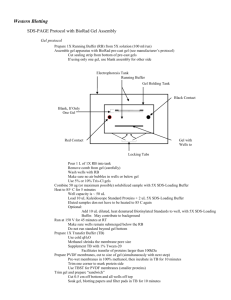
Western Blot Reagents: Lysis buffer (1X): 20 mM Tris pH 7.4 150 mM NaCl 1% Triton 100 5 mM EDTA 5 mM EGTA *Right before use, to 10 ml lysis butter add: 100 ul 0.1 M PMSF 10 ul 1 mg/ml leupeptin 5 ul 2 mg/ml aprotinin 10 ul 0.2 M Na3VO4 SDS-PAGE sample buffer (5X): 10% SDS 20 % Glycerol 0.2 M Tris pH 6.8 0.05% Bromophenolblue *For reducing gel, also add 10 mM beta-mercapto-ethanol SDS-PAGE running buffer (10X): 0.25 M Tris base 1.92 M glycine 1% SDS for 1L 30 g 144 g 10 g Transfer buffer (1X): 25 mM Tris base 192 mM glycine 20% methanol for 1L 3g 14.4 g 200 ml Blocking buffer (1X): 5% non-fat milk in TBST or 5% BSA in TBST *Antibodies should be diluted in either 5% non-fat milk in TBST or 3% BSA in TBST *Only 5% BSA should be used when PY20 is the primary antibody TBS (10X): 250 mM Tris base 1.37 M NaCl 27 mM KCl adjust pH to 7.4 for 1L 30 g 80 g 2g TBST (1X): for 1L 1X TBS 0.05% Tween 20 100 ml 10X TBS 2.5 ml 20% Tween 20 TBST for PY20 (1X): 25 mM Tris Base 100 mM NaCl 0.5% Triton adjust pH to 7.4 for 1L 3g 5.85 g 2.5 ml 20% Tween 20 Making cell lysate: Cell lysate should be kept cold at all time! From adherent cells: 1. Decant medium from 10cm dish of adherent cells and rinse plate rapidly with cold PBS. 2. Aspirate excess PBS. 3. Add 1ml cold lysis buffer, scrape cells from dish, transfer to a micro centrifuge tube, vortex and incubate on ice for 5 minutes. 4. Centrifuge the sample at 12,000 rpm for 15 minutes at 4oC. Transfer the supernatant to a new tube and store at -20oC. From cells in suspension: 1. Spin down cells and wash once with cold PBS. 2. Aspirate excess PBS and tap the tube gently to loosen up the cell pellet. 3. Add 1ml cold lysis buffer, mix by pipette up and down, vortex and incubate on ice for 5 minutes. 4. Centrifuge the sample at 12,000 rpm for 15 minutes at 4oC. Transfer the supernatant to a new tube and store at -20oC. Perform BCA protein assay: (optional) Run SDS-PAGE gel: 1. Transfer 20 ul of cell lysate to a new micro centrifuge tube. 2. Add 5 ul 5X SDS-PAGE denaturing sample buffer and mix well. 3. Boil for 5 minutes in a boiling water bath and spin briefly to collect all the liquid to the bottom of the tube. 4. Set up the SDS-PAGE apparatus and make sure it does not leak. 5. Load the samples. Make sure to run the Rainbow Marker (10 ul) together with the samples. 6. Run stacking gel under 100V, run separating gel under 200V (maximum current) until the blue dye front is about 1 cm to the bottom. Semi-dry Transfer: Wear gloves when handling the PVDF membrane! 1. Cut the membrane into 10X7.5 cm pieces. 2. Soak the membrane in methanol for 1 minutes and pour the methanol into a 50ml centrifuge tube for reuse. 3. Soak the membrane in transfer buffer for at least 5 minutes. Make sure the membrane does not float on top of the buffer. 4. Dissemble the SDS-PAGE gel apparatus. Cut off the stacking gel and the edge at the bottom of the gel. 5. Soak the gel in transfer buffer for 5 minutes. (Do not soak the gel for too long! The protein will diffuse.) 6. Set up the transfer sandwich (pad/gel/membrane/pad). Make sure there is no air bubble! 7. Transfer under 90mA (1.2 mA/cm2 of membrane) for 1 hour 45 minutes or 112.5 mA (1.5 mA/cm2 of membrane) for 1 hour. Western Blotting: 1. Dissemble the transfer apparatus and put the membrane protein side up in blocking buffer immediately. 2. Incubate at room temperature for 1 hour or 4oC overnight. (Make sure the membrane is submerged.) 3. Decant the blocking buffer and incubate in primary antibody at room temperature for 1 hour or 4oC overnight. 4. Wash 5x5 minutes with TBST. 5. Incubate in secondary antibody at room temperature for 30-60 minutes. 6. Wash 5x5 minutes with TBST. 7. Mix equal volume of ECL reagent I and II. Cover the membrane with the mixture for 1 minutes. 8. Rap the membrane with plastic rap and put on film. * When PY20 is used as primary antibody, use low salt TBST for all steps and only 5% BSA should be used for blocking and for diluting antibody