Unit V – Western Blot for Tumor Suppressor p53 in C2C12 and
advertisement

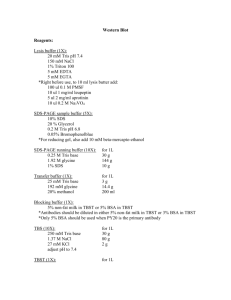
Unit V – Western Blot for Tumor Suppressor p53 in C2C12 and BC3H1 Cells The purpose of this unit is to learn the fundamentals of Western analysis, and to learn something about the characteristics of the tumor suppressor p53. You will determine which cell line, C2C12 or BC3H1, has levels of p53 that are detectable by Western analysis. One of these two cell lines expresses normal (wild type) p53 protein and the other expresses a mutant form of the protein. Based on your results and what you learn about p53 in lecture and your reading, you will determine which line expresses what form. Reading: Lecture material PowerPoints Cancer and the Cell Cycle; Monoclonal and Polyclonal Antibodies; SDS PAGE and Western analysis; p53 Technical manual for electrophoresis chamber - online Bio-Rad manual for Western blotting - online Amersham manual for ECL detection - online This handout for Western analysis May 20 Introduction Lecture on SDS Page & Western Lecture on Cancer and the Cell Cycle May 25 Run Western gel of BC3H1 and C2C12 cell extracts (both the stacking and resolving parts of the gel will be poured for you). Electroblot Lecture on monoclonal vs. polyclonal antibodies May 26 Take down electroblot and store (done for you) May 27 Block Western membrane (done for you before class) Probe Western with antibody; Detection Lecture on p53 14;18 abstract due as hard copy June 1 Analyze Western results Finish lectures Work on independent assignments June 3 Oral reports from independent assignment June 4, Midnight p53 Western Abstract and Midterm Recovery due by email. Email subject: MoDi Assignments Document titles: Lastname_p53 Lastname_Recovery Unit V: Polyacrylamide Gel Electrophoresis of Cell Extracts and Electroblotting Supplies: screw-cap microcentrifuge tubes discontinuous denaturing polyacrylamide gel: 10% polyacrylamide running gel, 5 cm long; 4% stacking gel (all 36.5:1-acryl.:bis and 0.15 cm thick) electrophoresis apparatus power supply electroblotting apparatus Stir bars, ice packs blotting paper nitrocellulose membrane plastic paper protector sheets plastic wrap aluminum foil zip-lock bag boiling water bath with floater Reagents: Cell extract samples of known total protein concentration, C2C12 and BC3H1 Kaleidoscope protein molecular weight standards (Bio-Rad) or equivalent RIPA buffer: 50mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 1 mM dithiothreitol; protease inhibitors: 1mm PMSF, 10 ug/ml leupeptin, 20 ug/ml aprotinin; phosphatase inhibitors – 1 mM NaF, 0.1 mM Na3VO4. 3X loading buffer: 175 mM TrisHCl, pH 6.8; 300 mM DTT; 5% SDS; bromphenol blue, 12 mg/ml; 18% glycerol Tris-glycine electrophoresis running buffer (pH 8.3): 25 mM Tris-base, 192 mM glycine, 1% SDS Tris-glycine transfer buffer (pH 8.5): 25 mM Tris-base, 192 mM glycine, 20% methanol, 0.01% SDS Unit V, cont’d. Protocol: Work with two teams of two per gel and electroblot apparatus. The gel has been poured for you. Be sure that you are aware of the gel composition and buffer components and understand their importance. 1. Preparation of samples Total cell extracts have been made from cultures of C2C12 cells and BC3H1 cells. Cells were grown as attached cultures on treated plastic mammalian cell culture plates. Cells were lysed and scraped off the plate with RIPA buffer + protease and phosphatase inhibitors. The lysate was transferred to a microfuge tube and vortexed to further disrupt the cell membrane, disrupt the nuclear envelope, and shear the DNA. The sample was subjected to centrifugation to pellet the cellular debris, and the supernatant, containing all soluble and solublized proteins, was frozen at -80oC for later analysis. The concentration of proteins in the extract was determined spectrophotometrically by the Bradford colorimetric method. A. You will be given a sample of extract from each cell type. Each sample you prepare should contain exactly 60µg total protein. Consult the board to determine the concentration of your sample, and from that, determine how many µl you will need to use. i. From the concentrations given, determine the volumes of each of your samples. BC3H1 = _______µl C2C12 = _______µl ii. Work on ice. For each of your four samples per group of 4, combine as follows in a screw cap tube: Sample volume RIPA buffer so that sample + RIPA = 20 µl 3X loading buffer Final volume = _______ _______ 10 µl 30 µl iii. For the Rainbow Markers (one sample/group of 4) 10 µl markers 10 µl H2O 10 µl 3X loading dye iv. Place the extracts from the cell lines into a boiling water bath for 10 minutes. (Do not put the Rainbow Markers into the water bath.) Plunge the samples into ice, quick-spin once the tubes have cooled, and load the gel as soon as possible. Unit V, cont’d. 3. Gel electrophoresis Flush out the wells as you did for the SSCP. Load the samples left to right as follows: Lane 1 – empty Lane 2 – BC3H1 extract from the first team Lane 3 – C2C12 extract from the first team Lane 4 – Rainbow markers Lane 5 – empty Lane 6 – empty Lane 7 – BC3H1 extract from the second team Lane 8 – C2C12 extract from the second team Lanes 9,10 – empty Make sure running buffer is above the wells and stays above the wells. Run the gel at 150V until the orange protein marker has moved to the very bottom of the gel. This will probably take about 70-90 minutes. 4a. Get the transfer sandwich materials ready for transfer. Refer to pages 5 and 6 of the Trans-blot PDF. Cut 2-4 sheets of Whatmann blotter paper to just slightly smaller than the fiber pads. (Note: this instruction is different from what you read in the pdf. We need more sheets to compensate for the compressed condition of the fiber pads, which they have acquired from use over time. 4b. When the electrophoresis is complete, set up the transfer as described in the pdf from Bio-Rad. Be sure to prewet all components of the sandwich, the fiber pads, the filter papers, and the membrane. To separate the electrophoresis plates, STOP and listen to verbal instructions that will depend upon whether your gels are commercially or in-house prepared. Float the gel loose from the second plate in a pan of running buffer, NOT transfer buffer. Next, equilibrate the gel very briefly in transfer buffer, and then for 5-10 minutes in fresh transfer buffer. Make sure the gel has completed any shrinkage. Be sure to place your gel into the transfer apparatus in the same orientation in which you ran it. Label the membrane with team name and mark left and right. Note: the labels must fall within the area of the gel itself! Label your transfer tank so that the person who takes down the blots in Step 5 will know how to label the membranes before storing them. Be sure you have connected the electrodes correctly! Run the transfer at 25V, overnight, in the cold room. 5. (The following steps will be performed by an instructor.) Take down the transfer as described in the pdf. Cut the membrane to the size of the gel. Mark where the colors are. Rinse the blot two times with 1X TBST. Store in plastic paper holder. Wrap the paper holder with plastic wrap. Wrap with foil. Store at 4oC. Unit V, cont’d. Western Analysis Supplies plastic boxes small for probing, larger for rinsing nitrocellulose membranes platform rocker plastic sheet protector autoradiography cassette X-ray film powder-free gloves Reagents TBST: 25mM Tris-HCl, pH 7.6, 125 mM NaCl, 0.1% Tween 20 Blocking solution: TBST + 5% non-fat dry milk Primary antibody (Hybridoma supernatant from cells making Pab421) Secondary antibody: Horse-radish peroxidase conjugated secondary antibody – anti-mouse IgG (from Amersham ECL kit) ECL (enhanced chemiluminescence) substrate, Detection Reagents #1 and #2 (Amersham) Odd-numbered teams will use primary antibody and secondary antibody; even-numbered teams will do all the steps, but will leave primary antibody out. Leaving primary antibody out is a control for the appearance of signal that results from non-specific binding of the secondary antibody. 1. Blocking – start blocking an hour before class. In small plastic box, cover the membrane with 10-15 mls blocking solution. Place on the rocker for 50-60 minutes at room temperature (before class). 2. +Primary antibody/ -Primary antibody Pour off the blocking solution. Lift the membrane out and cut it right down the middle of the rainbow markers. Replace one half in the box, and place the other half in a second box. a. and b. (Odd-numbered teams) Replace the blocking solution with 5 mls of blocking solution. Tip the box so one corner is down, add 50 ul of Anti-p53 Pab421 supernatant and mix. (This results in a 1:100 dilution of the supernatant.) (Even-numbered teams) In the second small plastic box, add 5 mls blocking solution with no antibody added. Incubate with rocking 50-60 minutes at room temperature. 3. In the larger box, rinse the membrane halves together quickly with 25 mls TBST. Repeat. 4. Rinse the membrane halves together twice with 50 mls TBST, 10 minutes per rinse. These rinses remove excess primary antibody. During this time, thoroughly rinse your small boxes with distilled water and dry. 5. Put 5 mls blocking solution into one clean small box. Add 1 ul (results in a 1:5000 dilution) horseradish peroxidase-conjugated secondary antibody. Submerge the membrane halves in the blocking solution + secondary antibody. Incubate for one hour at room temperature with rocking. Check periodically to keep the halves from overlapping. 6. In a larger box, rinse the membranes quickly two times, each time with 25 mls TBST. 7. Rinse the membrane three times, 10 minutes each, with 50-75 mls TBST. Steps 8-10 will be performed with two groups of 4 at a time in the dark room. Be sure you have read the Amersham ECL Detection directions as background for the following steps. One member of the class should precut a plastic sheet protector to fit the autoradiography cassette exactly and should prelabel it with fluorescent tape at a couple corners or edges on the inside bottom leaf for orientation. 8. Pour off the TBST. Do not leave excess TBST on the blot. Pick up the blot with forceps and suspend it against the side of the box for 30-45 seconds to drain excess TBST. Place the blot, protein side up, on a sheet of plastic wrap which you have torn from the roll. 9. Wear powder-free gloves. Make ECL detection reagent. Add 0.5 mls Detection Reagent #1 to 0.5 mls Detection Reagent #2 in a 1.5 ml microcentrifuge tube and mix. Using the P1000 micropipettor, add 0.5 to 0.75 mls of ECL detection reagent drop by drop to each of the half size blots. Cover them completely with detection reagent. Do not shake the blots. Incubate for one minute. After one minute, drain off excess detection reagent by picking up the blot with forceps and suspending it against the side of the box for 30-45 seconds. Smooth the membranes between the sides of the precut and labeled plastic sheet protector. It will be easiest to interpret the results if the two halves of an original blot are aligned to resemble the original single blot. Placing the blots asymmetrically also helps realign the developed film to the samples. 10. Expose the film, first for 30 minutes with double film, and then if necessary, overnight with single film.