Protein Extraction and Visualization
advertisement

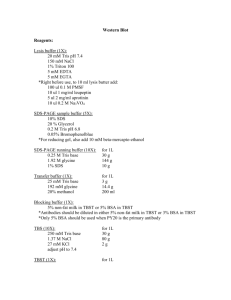
Protein Extraction for myc-tagged lines 1. Grind the equivalent of 20 continuously light-grown 6 day-old seedlings in 80 µL sample buffer. 2. Heat 95ºC for 3 min. 3. Bring to room temp, 5 min. 4. Spin down cellular debris 3 min, remove supernatant to new tube. Freeze -20ºC for up to 1 month. 5. Load 20 μL sample per lane on acrylamide gel. (If previously frozen, heat and spin again.) Sample Buffer, 1X Stock solution Volume 1 M Tris, pH 6.8 625 µL SDS, 20% 1 mL Glycerol, 100% 1 mL ß-mercaptoethanol 500 μL aliquot and store at -20ºC Final concentration 62.5 mM 2% 10% 5% Proteins can be quantified by using 1 μL sample + 800 μL 1 mM Tris, pH 6.8 + 200 μL BioRad protein assay dye reagent concentrate using 5, 10, 15, 20, & 25 μg/mL BSA standards. Immunoblotting 1. Remove stacking gel and equilibrate gel for 30 min in transfer buffer. 2. Measure gel and cut Whatman paper and NitroPure membrane to its size. 3. Pre-wet membrane 1 min in ddH20, then 15 min in transfer buffer. 4. Assemble transfer sandwich without any bubbles, pre-wetting sponges and Whatman paper first in transfer buffer. Order: Cassette top (red electrode) Sponge 3MM Whatman paper Membrane (NitroPure) Gel 3MM Whatman paper Sponge Cassette bottom (black electrode) 5. Fill tank with transfer buffer and ice block. Buffer should cover the electrode panels but should not touch the base of the banana plug. 6. Transfer proteins for 30 min - 1 h at 100 V with cooling, or for high MW proteins 14 V overnight. 7. Turn off power supply and disassemble the apparatus. Immunoprobing (using Pierce kit) 1. Block membrane. Incubate 1 h at room temp (or 4ºC overnight) in 10 mL blocking buffer, gently rocking: 2. Add 15 µL α-myc antibody (1:1000 stock kept in aliquots at -20ºC, 0.2 mg/mL). Incubate 1 h at room temp with gentle shaking. 3. Wash 5 times in 50 mL PBS-T for 5 min each. Visualization (in dark room) 1. Mix equal volume of luminal/enhance solution and stable peroxide solution (make 3 mL per blot). 2. Incubate blot for 5 min at room temp. 3. Remove the blot to a plastic sheet protector (press out bubbles). 4. Expose blot for 2 min to start (adjust later exposures up to 10 min). 5. Develop film and re-expose if necessary. Blocking Buffer PBS 1 X PBS 11.5 g Na2HPO4 (anhydrous), 80 mM 0.05% Tween 20 (or Triton X-100) 2.96 g NaH2PO4, 20 mM 5% Non-fat Dry Milk 5.84 NaCl, 100 mM QS to 10 mL, prepare fresh QS to 1 L, pH to 7.5, filter sterilize. Store 4ºC. PBS-T is PBS + 0.05% Tween-20