Review Activity
advertisement

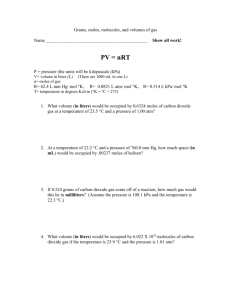
Review Activity Gases 1. According to the Kinetic-Molecular Theory, particles of matter are always in this. (motion) 2. According to the kinetic-molecular theory, this is what causes pressure exerted by gases (collision of particles with container walls) 3. According to the kinetic-molecular theory, the kinetic energy is directly proportional to this (Kelvin temperature of the gas) 4. This is the type of collisions that ideal gases have (elastic collisions) 5. During elastic collisions, none of this is lost (kinetic energy) Ideal gases 1. In ideal gases, there are none of these, but they do occur between real gas particles. (attractive forces) 2. Gas behavior is most ideal when the pressure is this way (low) 3. Gas behavior is most ideal when the temperature is this way (high) 4. Gas behavior is most ideal when the particles are like this (nonpolar) 5. In the ideal gas law, if the pressure is measures in atmospheres, this is the number for the ideal gas law constant (0.0821) Terminology: 1. The movement of one material through another material (diffusion) 2. The amount of force per given unit of area (pressure) 3. Tool that is used to determine atmospheric pressure (barometer) 4. What is standard at sea level (pressure) 5. The passing of gas molecules through a tiny opening in a container (effusion) Gas laws: 1. The law that says the spreading of gas molecules through a small opening is inversely proportional to the square root of its molar mass. (Graham’s Law) 2. Law that says the pressure and temperature are directly related (Gay-Lussac’s) 3. Law that says the temperature and volume of a gas are directly related (Charles) 4. According to Avogadro’s law, two things are related how? (Volume and number of moles are directly related). 5. According to Boyle’s law, two things are related how? (Pressure and volume are indirectly related) Miscellaneous: 1. One atmosphere of pressure is this many mmHg. (760) 2. If a gas has a temperature of 15.0 C, what temperature would you use in the combined gas law? (288 K) 3. What temperature is known as absolute zero? (O Kelvin or -273 Celsius) 4. One atmosphere of pressure is this many kPa of pressure (101.325) 5. If 32.00 grams of oxygen is used in an ideal gas situation, how many moles of gas will be used in the equation? (2) Problems: 1. A container holds oxygen, nitrogen and carbon dioxide gases. The total pressure of the mixture is 850 mmHg. If the nitrogen exerts 725 mmHg and the oxygen exerts 100 mmHg of pressure, how much pressure does the carbon dioxide exert? (25 mm Hg) 2. Carbon dioxide and bromine gas are in the same container. Determine the relative rate of effusion of the carbon dioxide compared to the bromine gas. (rate of CO2 is 1.906 times faster than Bromine’s rate) 3. A molecule of oxygen gas has an average speed of 12.3 m/s at a given temperature and pressure. What is the average speed of hydrogen molecules at the same conditions? (velocity hydrogen 49.0 m/s) 4. On the surface, where the pressure is 1.00 atm and the temperature is 20.0 Celsius, his gas tank of air has a volume of 5.0 L. When the diver goes 20.0 m, where the pressure is 3.00 atm and the temperature is 5.00 Celsius, what will the volume of air in his tank be? (1.6 L) 5. A balloon has a volume of 5.00 m 3 when it is at 25.0 Celsius. If the pressure stays the same, what volume will it expand to when it is heated to 50.0 Celsius? 5.42 m3 More problems: 1. An Easter “peep” has a volume of 0.75 cm 3 when it has a pressure of 1.0 atm on it. When it is placed in a vacuum tube and the pressure is decreased to 0.25 atm, what will its volume change to? (volume 3.0 cm 3 ) 2. A balloon has 1.25 moles of gas in it. If the temperature and pressure are to remain constant while 2.25 more moles are added to it, what will happen to the volume of the balloon? (The volume of the balloon will be 2.8 times bigger than the initial volume) 3. A gas is under 1.75 atm of pressure when its temperature is 75.0 C. If the volume stays the same, but the temperature decreases to 35.0 C, how many kPa of pressure will the gas be under? (157 kPa) 4. If 26.0 grams of Nitrogen dioxide are places in a container with a volume of 3.00-L and a temperature of 45.0 Celsius, what will the pressure of the gas be in mmHg? (3740 mm Hg) 5. A Nitrogen gas has 125 kPa of pressure and a temperature of 35.0 Celsius with a volume of 44.5 L. What mass of Nitrogen gas is in the container? (60.8 g) All Ideal/Stoichiometry: 1. Hydrogen peroxide decomposes to Oxygen and water. How much mass of hydrogen peroxide is needed to obtain 0.325 L of oxygen at 10.0 Celsius and 1.25 atm? (1.19 g) 2. When nitrogen and hydrogen gases react, they can form ammonia (NH 3 ). How many liters of Nitrogen can be formed from 22.0 grams of ammonia at 12.0 C and 1.23 atm of pressure? (12.3 L) 3. Nitrogen and oxygen gases react at STP to form nitrogen dioxide. If you start with 15.4 L of nitrogen and an excess of oxygen gas, how much nitrogen dioxide would be formed? (1.38 moles or 63.3 g) 4. Calcium Carbonate decomposes to form Calcium oxide and carbon dioxide. If 1.37 L of carbon dioxide are formed at 105 kPa and 35.0C, how many grams of Calcium carbonate did you start with? (5.63 g) 5. If an excess amount of Aluminum combines with 18.0-L of oxygen at 1.03 atm and 19.9C to form Aluminum oxide, how many grams of aluminum oxide are formed? (52.4 g) Questions without Answers Gases 1. According to the Kinetic-Molecular Theory, particles of matter are always in this. 2. According to the kinetic-molecular theory, this is what causes pressure exerted by gases 3. According to the kinetic-molecular theory, the kinetic energy is directly proportional to this 4. This is the type of collisions that ideal gases have. 5. During elastic collisions, none of this is lost. Ideal gases 1. In ideal gases, there are none of these, but they do occur between real gas particles. 2. Gas behavior is most ideal when the pressure is this way. 3. Gas behavior is most ideal when the temperature is this way. 4. Gas behavior is most ideal when the particles are like this. 5. In the ideal gas law, if the pressure is measures in atmospheres, this is the number for the ideal gas law constant. Terminology: 1. The movement of one material through another material. 2. The amount of force per given unit of area. 3. Tool that is used to determine atmospheric pressure. 4. What is standard at sea level. 5. The passing of gas molecules through a tiny opening in a container. Gas laws: 1. The law that says the spreading of gas molecules through a small opening is inversely proportional to the square root of its molar mass. 2. Law that says the pressure and temperature are directly related. 3. Law that says the temperature and volume of a gas are directly related. 4. According to Avogadro’s law, two things are related how? 5. According to Boyle’s law, two things are related how? Miscellaneous: 1. One atmosphere of pressure is this many mmHg. 2. If a gas has a temperature of 15.0 C, what temperature would you use in the combined gas law? 3. What temperature is known as absolute zero? 4. One atmosphere of pressure is this many kPa of pressure 5. If 32.00 grams of oxygen is used in an ideal gas situation, how many moles of gas will be used in the equation?