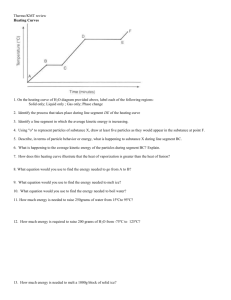
Grams, moles, molecules, and volumes of gas
advertisement

Grams, moles, molecules, and volumes of gas Name _____________________________________________ Show all work! PV = nRT P = pressure (the units will be kilopascals (kPa) V= volume in liters (L) n= moles of gas (There are 1000 mL in one L) R= 62.4 L mm Hg/ mol °K, R= 0.0821 L atm/ mol °K, R= 8.314 L kPa/ mol °K T= temperature in degrees Kelvin (°K = °C + 273) 1. What volume (in liters) would be occupied by 0.6324 moles of carbon dioxide gas at a temperature of 23.5 °C and a pressure of 1.00 atm? 2. At a temperature of 22.2 °C and a pressure of 760.0 mm Hg, how much space (in mL) would be occupied by .00237 moles of helium? 3. If 0.524 grams of carbon dioxide gas come off of a reaction, how much gas would this be in milliliters? (Assume the pressure is 100.1 kPa and the temperature is 22.3 °C.) 4. What volume (in liters) would be occupied by 6.022 X 1023 molecules of carbon dioxide gas if the temperature is 23.9 °C and the pressure is 1.01 atm? 5. Suppose you have 0.420 grams of hydrogen gas (H2) come off a reaction and you collect the gas in a cylinder. In theory, how many liters of gas should you be able to collect if the temperature is 22.2 °C and the pressure is 760.2 mm Hg? 6. If you have 2.1 X 1023 helium atoms, how many liters of helium do you have? (Assume a temperature of 23.5 °C and a pressure of 100.0 kPa? 7. Before an alka-seltzer tablet is dropped into a glass of water, the glass of water and the alka-seltzer tablet are found to have a mass of 35.621 grams. After the tablet is dropped into the water, and all the carbon dioxide has bubbled out, the glass of water has a mass of 35.102 grams. In theory, how many milliliters of carbon dioxide gas bubbled out of the water? (Assume a temperature of 21.5 °C and a pressure of 1.02 atm? 8. Before a can of soda is opened it has a mass of 712.886 grams. The can is opened and is left open at room temperature for several hours. The can of soda is massed again and now has a mass of 712.224 grams. Assuming that carbon dioxide gas was the only thing that left the soda, how much carbon dioxide escaped in milliliters. (Assume a temperature of 25.0 °C and a pressure of 760.0 mm Hg) Answers: 1. 15.4 L CO2 5. 5.05 L H2 2. 57.4 mL He 3. 292 mL CO2 4. 24.1 L CO2 6. 8.60 L He 7. 280. mL or 2.80 X 102 mL CO2 8. 368 mL CO2