chemistrycrosswordclues
advertisement

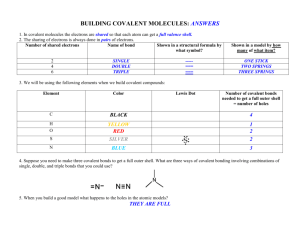
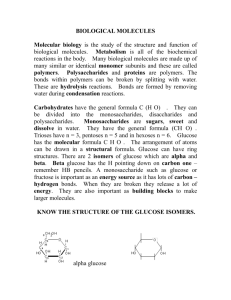
ACROSS 12 The name given to a polymer of amino acids; equivalent to protein 13 Functional group of many organic acids 15 Number of covalent bonds for each oxygen 17 A covalent bond where the electrons are not shared equally among the bonded atoms 19 A fatty acid with one or more double bonds 21 Arrangement of phospholipids in cell membranes 22 A fatty acid with no double bonds 23 A disaccharide consisting of glucose and fructose 24 An atom that has gained or lost one or more electrons 28 Monomers bonded end to end in a long chain 30 The monomer of chitin 31 Descriptive of water-soluble, polar molecules 34 A reaction that breaks down molecules with the addition of a water molecule 36 Glycerol, 2 fatty acids, and a phosphate group; major component of cell membranes 37 Prefix meaning "two" 39 Sub-atomic particle in the nucleus with a zero charge 40 Dietary lipid with a glycerol residue bonded to 3 fatty acids 42 Solid, liquid, or gas that results in the dissociation of hydroxide ions 43 A chemical bond involving one pair of shared electrons 46 The 5-carbon sugar component of RNA 47 A reaction that joins monomers by removing a molecule of water 48 Number of covalent bonds for each hydrogen 50 The study of matter 52 A solution with liberated ions and electrons capable of conducting electric current 54 An alcohol with only one carbon 56 A covalent bond with electrons shared equally among bonded atoms 59 Different versions of elements owing to differing numbers of neutrons 60 A polymer of amino acids 62 Function group (COOH) that gives up a proton in aqueous solutions; acidic 64 A polymer of glucose common to plants 65 A disaccharide consisting of glucose and galactose 66 Number of covalent bonds to each oxygen 67 A polymer of glucosamine found in arthropod exoskeletons and fungal cell walls DOWN 1 The covalent bond between amino acids 2 The most polar of all molecules in biological systems; the liquid of life 3 An alcohol with 2 carbons 4 The 3d structure of proteins based on chemical and polar interactions between amino acid side chains 5 The level of protein structure based on hydrogen bonding between amino acid side chains 6 Descriptive of fat-soluble, non-polar molecules 7 A polymer of glucose found in the cell walls of all plants 8 A polymer of glucose found in animal cells 9 The element common to organic molecules 10 The science of life 11 Prefix meaning "many" 14 Number of covalent bonds to each hydrogen 16 Suffix for all hydrocarbons with only single bonds between the carbons 18 The level of protein structure based on the sequence of amino acids 20 Technical term for sugar 25 Suffix for molecules containing at least one hydroxyl group 26 The monomer of protons 27 The 5-carbon sugar of DNA 28 The measure of the acidity of a solution 29 Class of organic molecules with a C:H:O ration of 1:2:1 32 Number of covalent bonds to each carbon in a molecule 33 Number of covalent bonds for each carbon 35 Prefix meaning "one" 38 Negatively charged sub-atomic particle 41 Bond involving two pairs of shared electrons 42 The mascot of OHS 44 Function group that strips a proton from a water molecule, resulting in a dissociated hydroxide ion 45 Molecules with the same empirical formula, but with different structures and characteristics 49 Element with 5 valence electrons; component of proteins and nucleic acids 51 Function group with -OH 53 Class of non-polar molecules 55 Generic term for the units joined together in a polymer 57 The electrons outside a filled orbital 58 Class of organic molecules containing a single hydroxyl group 60 Positively charged sub-atomic particle in the nucleus 61 The scientific name of plant starch 63 Solid, liquid, or gas that results in a dissociated hydrogen ion