VIDEO TEST-REVERSE ALPHABET
advertisement

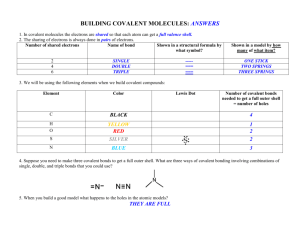
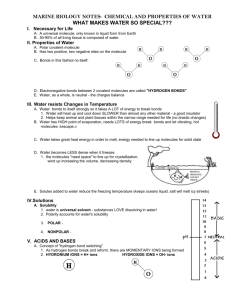
TEST Circle the phrase which best answers each question. 1. Compounds are formed when atoms give up, gain or share: • neutrons. • protons. • electrons. • photons. 2. _____ occur when electrons are transferred from one atom to another. • Ionic bonds • Covalent bonds • Noble gases • Ions 3. Most noble gases are stable elements because their electrons form: • hydrogen molecules. • covalent bonds. • ionic bonds. • octets. 4. The pH scale measures the relative strength of acids or bases in: • isolation. • metals. • solutions. • hydrocarbons. 5. When atoms combine with the help of covalent bonds, _____ are formed. • electrons. • neutrons. • protons. • molecules. 6. Molecules are represented using a shorthand method called: • oxidation numbers. • polyatomic formulas. • compound ions. • chemical formulas. 7. The ability of an element to combine with other elements is represented by its: • oxidation number. • chemical formula. • pH level. • compound number. 8. _____ are formed when molecules share electrons. • Metals • Acids • Hydrocarbons • Polyatomic ions 9. Methane is an example of: • a subatomic particle. • a hydrocarbon. • a covalent bond. • an octet. 10. Nearly all of the elements with negative oxidation numbers are: • metals. • gases. • organic chemicals. • halogens. REVERSE ALPHABET An important word in each sentence below is written in Reverse Alphabet. Use the code below to discover the important word in each sentence. A= Z, B=Y, C=X, D=W, E=V, F=U, G=T, H=S, I=R, J=Q, K= P, L = O, M=N, N=M, O=L, P=K, Q=J, R=I, S=H, T=G, U=F, V=E, W=D, X=C, Y=B, Z=A 1. The forces that hold atoms together in compounds are called XSVNRXZO bonds. _________________________ 2. Noble gases have very stable VOVXGILM configurations. _________________________ 3. The ZOPZORMV Earth Metals achieve a stable octet by losing one electron and gaining a positive ion. _________________________ 4. Compounds formed by covalent bonding are often ORJFRWH or TZHVH at room temperature. _________________________ 5. The combining ability of an element is described by its LCRWZGRLM number. _________________________ 6. Ions formed out of molecules are referred to as KLOBZGLNRX ions. _________________________ 7. All bases contain negatively charged SBWILCRWV ions. _________________________ 8. Organic compounds are formed through XLEZOVMG bonds. _________________________ © Copyright 1999 AIMS Multimedia Compounds: Electromagnetic Attraction in Molecules