Acid - Base notes - Southwest High School

Acid Base Intro notes 2015
Acids: start with “H”, make H + ions in solution with water
Examples: HCl, H
2
SO
4
, HF, H
3
PO
4
Nomenclature: hydrochloric acid, sulfuric acid, hydrofluoric acid, phosphoric acid
Taste sour, have a pH less than 7
Bases: end with “OH” make OH ions in solution with water
Examples: LiOH, NaOH, KOH, Al(OH)
3
Nomenclature: lithium hydroxide, sodium hydroxide, potassium hydroxide, aluminum hydroxide
Taste bitter, feel slippery (dissolve fats) have a pH greater than 7
Ammonia (NH
3
) is a type of base called a Lowry/Bronsted base: (get used to drawing “conjugate pairs”)
NH
3
+ H
2
O
NH
4
+
+ OH
-
There are 3 standard definitions of an ACID :
Arrhenius: start with “H” and produce H
+
ions in water
Lowry-Bronsted: are a proton (aka Hydrogen cation) donor
Lewis: electron pair acceptor
There are 3 standard definitions of a Base :
Arrhenius: end with “OH” and produce OH
-
(hydroxide) ions in water
Lowry-Bronsted: are a proton acceptors
Lewis: electron pair donor
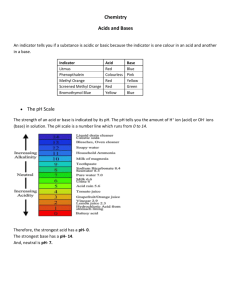
The concentration of H + is so critical in the rate of chemical reactions (and the equilibrium of the human body) that we have a special scale for measuring tiny concentrations of the H + ion called the pH scale. pH means power of the hydrogen ion concentration
Mathematically pH is a function defined as: pH = - log [H + ]
(pH is the negative of the log of the hydrogen ion concentration)
Logarithm (log) Review:
-log(.1) = 1
Exponent Review:
1x10
-1
= .1
1x10
-7
= .0000001
1x10
-14
= .00000000000001
-log(0.0000001) = 7
-log(0.00000000000001) = 14
(Practice pH, pOH and H
+
concentration on worksheet)
Acids and Bases react together and NEUTRALIZE to produce salt and water
Acid + Base
salt + water (not just table salt, any salt)
Acids:
Dissolve metals, (producing H
2
gas) react with carbonates to produce bubbles, low (1-7) pH,
Must have hydrogen (often have oxygen as well), turns Litmus paper RED, taste SOUR
When they ionize they produce H
+
ions which combine with water to make the H
3
O
+
(hydronium ion)
Bases:
High pH (7-14), good for cleaning (like ammonia), turn litmus paper BLUE, taste bitter feel slippery
Bases ionize to produce OH
-
(hydroxide ions)
Indicators: are a class of chemicals that undergo a visible color change in chemical environments that have a greater abundance of H
+
or OH
-
ions.
Some important indicators:
Bromthymol Blue:
Phenolphthalein
Blue in basic conditions violet in basic conditions yellow in acids clear in neutral or acid
Anthrocyanin:
(aka Cabbage Juice
Indicator)
Cabbage juice indicator is _____blue/lavender__ in water (neutral)
Cabbage juice indicator is ____reddish pink________ in acids
Cabbage juice indicator is _____green__________ in bases
Universal indicator (a mixture of four separate indicators that include, bromthymol blue, phenolphthalein, and methyl red
ROY G BIV (know the colors this reminds us of)
R O Y G BIV The colors of visible light (in a rainbow) lowest energy or longest wavelength(
) to highest energy or shortest wavelength(
)
Some acids are poly-protic (means they can give up more than one hydrogen ion)
HNO
3
is monoprotic
H
2
SO
4
is diprotic
H
3
PO
4
is triprotic
Strong acids completely ionize (change from molecules to ions)
Would their K a
be greater than or less than 1?
Strong Bases completely dissociate (dissolve from their formula unit to their ions)
Would their K b
be greater than or less than 1?
Weak Acids and Bases will try to remain together and will covert from their equilibrium positions as stress is placed upon them (water is just as weak an acid as it is a weak base)
Acids are formed from reaction of non-metal oxides and water
Ex: SO
2
+ H
2
O
H
2
SO
4
(not balanced)
Or CO
2
+ H
2
O
H
2
CO
3
(demo with BTB)
Bases are formed from metal oxides and water
Ex: CaO + H
2
O
Ca(OH)
2
Or MgO + H
2
O
Mg(OH)
2
(demo; with universal indicator)
Neutralization occurs when the same number of Moles of acid reacts with an equivalent number of moles of base to stoichiometerically form a salt and water.
The equation to remember is: mL acid
M acid
= mL base
M base
(volume times concentration = volume times concentration)
Volume measured in milliliters and concentration measured in Molality (moles per liter)
A salt may hydrolyze (react with water) to form an acidic or alkali solution, depending upon its “parent” acid and base.
A parent acid and a parent base neutralize to for a salt that may be “acidic” like it’s parent acid ; basic (or alkali) like it’s parent base or neutral, equally acidic and alkali (more common)
Memorize these strong acids: HCl, HBr, HI,H
2
SO
4
, HNO
3
, HClO
3
(Chloric acid)HClO
4
(Perchloric acid)
and HC
2
H
3
O
2
or CH
3
COOH as acetic (or ethanoic acid aka vinegar) a very common weak acid
Memorize these strong bases: LiOH, NaOH, KOH, RbOH, CsOH, FrOH, Ca(OH)
2
, Sr(OH)
2
, Ba(OH)
2
,
Ra(OH)
2
the alkali metals and the soluble alkaline earth metals.
And the base NH
3
(ammonia) an important weaker base helps you understand Lowry-Bronsted conjugate pairs.
NH
3
+ H
2
O
NH
4
+
and OH
-
(NH
3
and OH are the bases; H
2
O and NH
4
+ are the acids)
Recap of titration calculations:
Complete neutralization occurs when there is just as much acid as there is base
For mono protic acids and mono hydroxyl bases
Volume of acid times the concentration of the acid will equal the volume of the base times the concentration of the base
Or more simply: mL acid
M acid
= mL base
M base
(milliliters of acid times the molarity of the acid equals the milliliters of base times the
Molarity of the base)
How many ml of .25 M HCl would be needed to neutralize 30.0 ml of .15 M NaOH?