Acids and Bases Worksheet
advertisement

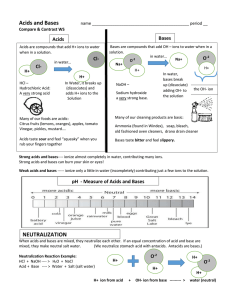
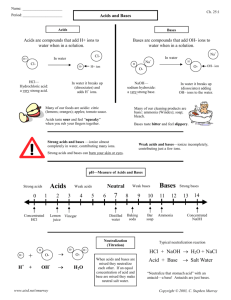
Name:_____________________________ Date:___________ Class:______________ What do You Know About Acids and Bases? Letter Matching 1. Acid A. To mix acids and bases to cancel each other out and make water and salt B. A compound that adds H+ ions to water 2. Base 3. Neutral 4. Neutralize C. Equal number of H+ and OH- ions; water is an example D. A compound that adds OH- ions to water. 5. Acid Rain E. When pollution causes rain to have a pH less than 5.6. 6. pH F. The measure of how acidic or basic a solution is 7. Salt Water G. A compound that adds a few OH- ions to water 8. Strong Acid H. The product of a neutralization reaction between an acid and a base 9. Weak Base I. A compound that adds a few H+ ions to water 10. Weak Acid J. A compound that adds a lot of H+ ions to water. Is it an Acid or a Base? Circle the acids and underline the bases 11. HCl 17. H2CO3 23. apple juice 12. Mg(OH)2 18. NaOH 24. lemonade 13. H3PO4 19. Al(OH)3 25. soap 14. KOH 20. HBr 26. laundry detergent 15. Ca(OH)2 21. H2SO4 27. soft drinks 16. LiOH 22. H2O 28. bathroom cleaner Acids or Bases? Identify the following with an A for Acid or B for Base 29. pH of 1 to 7 30. Feels slippery 31. pH of 7 to 14 32. Has more OH- ions 33. Has more H+ ions 34. Tastes sour Acid-Base Practice 1 A student wearing goggles, gloves, and an apron begins a simple activity to determine the pH of corrosive solutions. Before the activity, what other safety measures should the student follow? A Identify the locations of eye wash, shower, and fire equipment B Check and set clocks and record the beginning time C Review the proper method of fire-polishing glass tubing D Arrange the equipment in the work area alphabetically 2 Hard water has a pH higher than 7 and an abundance of calcium and magnesium salts. Which of the following would be the best cleaning solution for removing hard-water residue from drinking glasses? A A mild acid such as vinegar B A strong solution such as ammonia C A hot solution such as baking soda in boiling water D A strong base such as sodium hydroxide 3 Two clear solutions are placed in separate beakers. The first solution has a pH of 4, and the pH of the second solution is unknown. If the two solutions are mixed and the resulting pH is 5, the second solution must have – A fewer suspended solids B a lower temperature C more dissolved salt (NaCl) particles D a higher concentration of OH-ions Properties of Some Solutions Solution Electrical Conductivity of Solution Original Color of Litmus Paper 1 2 3 4 Very high Low Moderate Very high Red Blue Red Blue Color of Litmus Paper After Dipping in Solution Blue Red Red Red pH 10.0 6.5 5.4 2.0 4 The table shows data from an investigation designed to find a liquid solution that is both an acid and a strong electrolyte. Based on the data, a solution that is both an acid and a strong electrolyte is – A Solution 1 B Solution 2 C Solution 3 D Solution 4 Venn Diagram Acids Bases