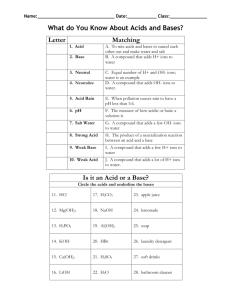
Acid/Base Review Guide
advertisement
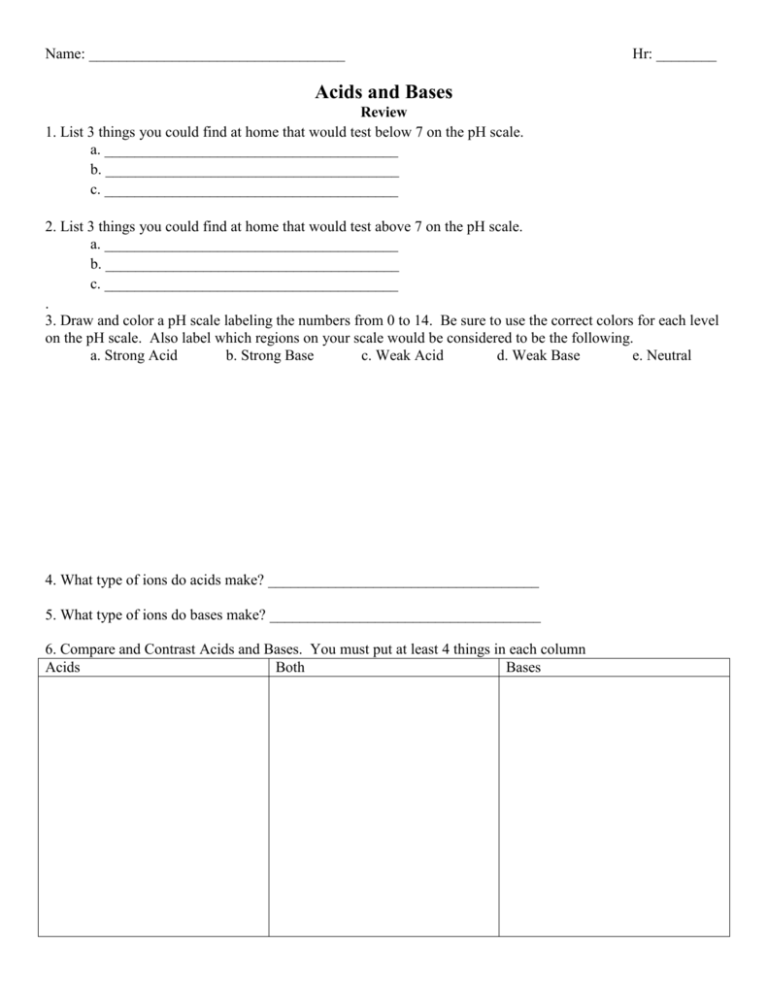
Name: __________________________________ Hr: ________ Acids and Bases Review 1. List 3 things you could find at home that would test below 7 on the pH scale. a. _______________________________________ b. _______________________________________ c. _______________________________________ 2. List 3 things you could find at home that would test above 7 on the pH scale. a. _______________________________________ b. _______________________________________ c. _______________________________________ . 3. Draw and color a pH scale labeling the numbers from 0 to 14. Be sure to use the correct colors for each level on the pH scale. Also label which regions on your scale would be considered to be the following. a. Strong Acid b. Strong Base c. Weak Acid d. Weak Base e. Neutral 4. What type of ions do acids make? ____________________________________ 5. What type of ions do bases make? ____________________________________ 6. Compare and Contrast Acids and Bases. You must put at least 4 things in each column Acids Both Bases Vocabulary 7. A (an) __________________________________ is an organic compound that changes color in the presence of an acid or base. 8. A (an) __________________________________ is a substance that produces hydroxide ions. 9. A (an) __________________________________ is a substance that produces hydronium ions. 10. A (an) __________________________________ acid/base is one that completely ionizes in a solution. 11. A (an) __________________________________ acid/base is one that only partially ionizes in a solution 12. The _________________________________ of an acid or base refers to the amount of acid or base dissolved in a solution. This is what we test using the pH scale. 13. Any rain that falls to the ground with a pH lower than ______________ is considered to be acid rain. 14. A (an) __________________________________ reaction is a chemical reaction between an acid and base creating water and salt. 15. Identify if we have an acid or base then complete the ionization reactions. Sr(OH)2 Acid or Base _____________________ ____________________ + _______________________ HI Acid or Base ____________________ + _______________________ ____________________ + _______________________ 16 Complete the following Neutralization Reaction. HBr + LiOH + H2O ___________ + ____________ + _____________ ___________+ ___________+__________+____________ Water: ___________ + ____________ ____________ Salt: __________+____________ ____________