Pharmacy Pearls - CU Family Medicine
advertisement

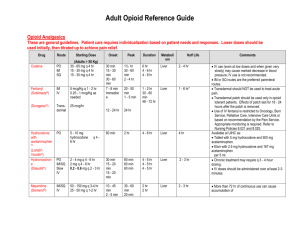
Pharmacology Pearls – Chronic Opioid Use Renal and Hepatic Dysfunction (www.practicalpainmanagement.com/treatments/pharmacological/opioids) General recommendations for opioids in patients with renal insufficiency Drug Name Meperidine Codeine Recommendation Not Recommended Not Recommended Morphine Use cautiously Oxycodone Use cautiously Hydromorphone Use cautiously Fentanyl Appears safe Methadone Appears safe General recommendations for opioids in patients with liver insufficiency Drug Name Meperidine Codeine Recommendation Not Recommended Not Recommended Morphine Use cautiously Oxycodone Use cautiously Hydromorphone Use cautiously Fentanyl Appears safe Methadone Not Recommended Notes Accumulation of toxic metabolite, normeperidine, may cause CNS toxicity. Impaired conversion of codeine to the active compound, morphine, in the liver to be active. Recommended to decrease frequency of administration and dosage because of decreased clearance and increased t1/2 and oral bioavailability. Risk of accumulation of parent drug due to decreased conversion to metabolites and decreased elimination. Recommended to reduce dose by 1/2 to 1/3 of the usual amount and avoid in severe cirrhosis. Risk of accumulation of parent drug due to decreased conversion to metabolites and decreased elimination. Recommended to decrease dose by 50% of the usual amount. Pharmacokinetics were not altered in patients with cirrhosis. With continued use, recovery time after termination of infusion may be longer. Risk of accumulation with severe liver disease. Fentanyl o o o Notes Accumulation of toxic metabolite, normeperidine, may cause CNS toxicity Accumulation of active metabolites in renal failure.5 Case reports of toxicity and serious adverse effects which can be delayed Metabolite morphine-6-glucuronide, a more potent analgesic, may accumulate causing more sedation.4 If morphine must be used, it is recommended to use cautiously and adjust dose appropriately Insufficient evidence available for strong recommendation. Reports of accumulation of both parent compound and active metabolite, free oxymorphone, leading to CNS toxicity and sedation. Metabolite, hydromorphone-3-glucuronide can accumulate and cause CNS toxicity. Careful monitoring of patients on dialysis and avoid use in patients with GFR < 30mL/min. Some reports of parent compound accumulating in renal failure, but no increase in adverse effects. The metabolites are considered inactive. A dose reduction may be necessary. With long term use, careful monitoring of pharmacodynamic effects is advised. Recommended to be used only by experienced clinicians. No active metabolites are formed and limited plasma accumulation of the drug observed in renal failure. Dose reduction may be necessary in severe renal failure. Never start in an opioid naïve patient Transdermal delivery offers unique benefits, but results in delayed action and may not last 72 hours in all patients Refer to product labeling for guidance on fentanyl conversions and use more conservative approaches than are typically used with long acting opioids Methadone o o o o o Too rapid of titration can result in unintentional overdoses Little cross tolerance with other opioids making conversations complicated Highly variable metabolism; must titrate slowly (no sooner than every 5 days) Consult with a provider who has experience with using methadone prior to use EKG testing needed due to Qtc prolongation, and might need to be avoided in high risk patients Red Flag Regimens Violating terms of provider-patient opioid agreement Use of more than one short acting opioid High quantity of short acting opioids per prescription in relation to the long acting opioid Patterns of early filling that are frequent or not explained Multiple providers of opioid prescriptions identified on PDMP reports Consider the Morphine Equivalent (ME) of an Opioid Regimen Evidence has convincingly demonstrated that higher opioid doses are associated with an increased risk of harm, specifically overdose and related mortality, bone fracture, and emergency department visits Morphine equivalent dosing is now an explicit criteria singled out by DORA for judging monitoring intensity and prescribing appropriateness. The increased risk of fatal overdose significantly increases in patients receiving opioid therapy consisting of 100-mg of morphine equivalents (ME) or higher. Per the state board “Opioid doses >120 mg morphine equivalents per day is a dosage that the Boards agree is more likely dangerous for the average adult (chances for unintended death are higher) over which prescribers should use clinical judgment, put in place additional safeguards for the treatment plan (such as utilizing a treatment agreement), consult a specialist or refer the patient; and dispensers should be more cautious.” Several calculators are available to allow clinicians to determine a patient’s ME Example Regimens: Patient 1: o Oxycontin (oxycodone SR) 20 mg po Q12h o Norco (hydrocodone/APAP) 10/325 mg po q4-6h prn (average 4 daily) Total daily use is equal to 100 ME Patient 2: o Dilaudid (hydromorphone) 4 mg 1 to 2 tablets q3-4h prn (average 7 daily) Total daily use is equal to 112 ME Patient 3: o MS Contin (morphine SR) 30 mg po Q12h o Roxicodone (oxycodone) 10 mg po q3-4h prn (average 4 daily) Total daily use is equal to 120 ME Naloxone Rescue for Overdoses (http://prescribetoprevent.org/wp-content/uploads/2012/11/one-pager_22.pdf, http://stopoverdose.org/docs/NaloxoneBrochure.pdf ) Intranasal Spray Given with a foam tip (nebulizer, adapter, or atomizer) that is put on a syringe then placed into the nostril Intranasal naloxone has not been approved by the FDA (i.e., it is an "off-label" delivery method), but can be legally prescribed by a physician or approved pharmacists First responders often give naloxone intranasally Intramuscular Injection Evzio™ is a 0.4 mg auto-injector that is commercially available (similar to an EpiPen ®)