Phase Change Notes
Name:_____________________________________________________________________________________________________________
Phase Change Notes (Unit 7.5)
Any substance has 3 types of energy:
1.
Thermal- we already know that thermal energy is how fast the particles are moving. When a substance gains or loses this type of energy it causes a change in the temperature. The faster the particles are moving the more thermal energy they have and the higher the temperature of the substance.
2.
Phase- This is the amount of energy a substance has due to the interactions of the particles. Phase energy decreases as the particles in a substance interact with each other. Substances in the solid phase have the least amount of phase energy and substances in the gas phase have the most.
3.
Chemical- Energy that is stored in the bonds between particles. We will discuss chemical energy more later.
Adding Energy to a System:
When a substance absorbs energy from its surroundings this energy can go into the thermal energy, phase energy and chemical energy “accounts.”
When energy gets added to the thermal account the particles of the substance move faster. Therefore, the temperature of the substance rises.
When the energy gets added to the phase account the particles of the substance are able to overcome the attractions between them so that they can move into another phase. In other words, when a solid absorbs energy into the phase account it can become a liquid, or a liquid could become a gas.
Substances absorb energy into the phase account only when they are at the melting and boiling point temperatures.
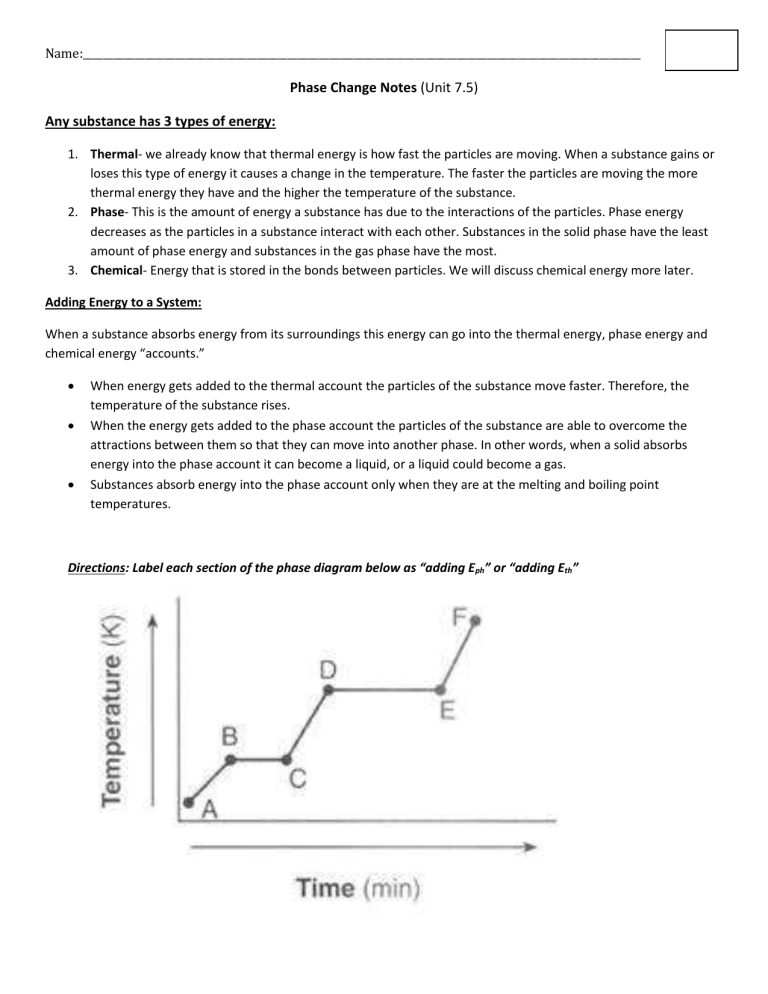
Directions: Label each section of the phase diagram below as “adding E ph
” or “adding E th
”
Name:_____________________________________________________________________________________________________________
Adding Energy to a System Cont’d:
When a substance is absorbing energy into its thermal account, it is not going through a phase change.
Therefore, when the temperature is increasing the substance exists in only one phase.
When a substance is accepting energy into its phase account, it is going through a phase change. Therefore when the temperature is not going up the substance is in two phases at once.
Directions: Label each section of the phase diagram on the previous page as” solid only”, “liquid only”, “gas only”,
“solid and liquid”, or “ liquid and solid.”
Removing Energy from a System:
When a substance releases energy to its surroundings this energy can come out of the thermal energy, phase energy and chemical energy “accounts.”
When energy gets removed from the thermal account the particles of the substance slow down. Therefore, the temperature of the substance decreases.
When the energy gets removed from the phase account the particles of the substance are no longer able to overcome the attractions between them so that they change into a less energetic phase. In other words, when a liquid releases energy from its phase account it can become a solid, or a gas could become a liquid.
Substances release energy into the phase account only when they are at the condensation and freezing point.
Directions: Label each section of the phase diagram below as “removing E ph
” or “removing E th
”
Name:_____________________________________________________________________________________________________________
Removing Energy from a System Cont’d:
When a substance is removing energy from its thermal account, it is not going through a phase change.
Therefore, when the temperature is decrease the substance exists in only one phase.
When a substance is removing energy from its phase account, it is going through a phase change. Therefore when the temperature is not decreasing the substance is in two phases at once.
Directions: Label each section of the phase diagram on the previous page as” solid only”, “liquid only”, “gas only”,
“solid and liquid”, or “ liquid and solid.”












