Phy 122 L_GasLaw
advertisement
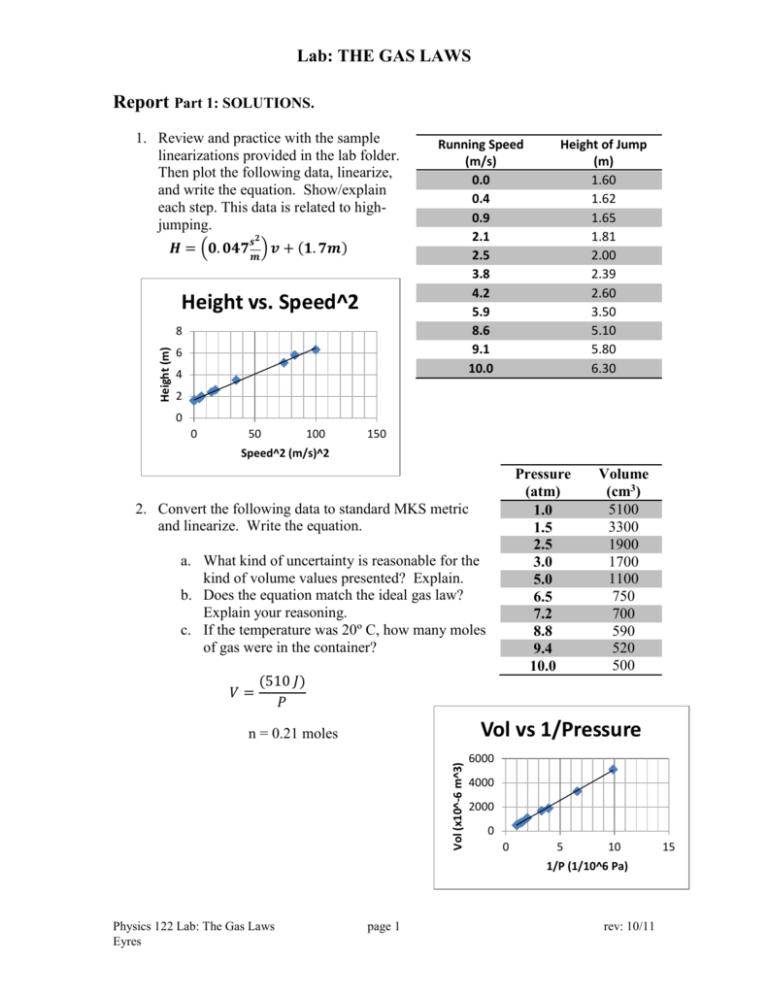
Lab: THE GAS LAWS Report Part 1: SOLUTIONS. 1. Review and practice with the sample linearizations provided in the lab folder. Then plot the following data, linearize, and write the equation. Show/explain each step. This data is related to highjumping. 𝒔𝟐 𝑯 = (𝟎. 𝟎𝟒𝟕 𝒎 ) 𝒗 + (𝟏. 𝟕𝒎) Height vs. Speed^2 Height (m) 8 6 4 Running Speed (m/s) 0.0 0.4 0.9 2.1 2.5 3.8 4.2 5.9 8.6 9.1 10.0 Height of Jump (m) 1.60 1.62 1.65 1.81 2.00 2.39 2.60 3.50 5.10 5.80 6.30 2 0 0 50 100 150 Speed^2 (m/s)^2 Pressure (atm) 1.0 1.5 2.5 3.0 5.0 6.5 7.2 8.8 9.4 10.0 2. Convert the following data to standard MKS metric and linearize. Write the equation. a. What kind of uncertainty is reasonable for the kind of volume values presented? Explain. b. Does the equation match the ideal gas law? Explain your reasoning. c. If the temperature was 20º C, how many moles of gas were in the container? 𝑉= Volume (cm3) 5100 3300 1900 1700 1100 750 700 590 520 500 (510 𝐽) 𝑃 Vol vs 1/Pressure Vol (x10^-6 m^3) n = 0.21 moles 6000 4000 2000 0 0 5 10 1/P (1/10^6 Pa) Physics 122 Lab: The Gas Laws Eyres page 1 rev: 10/11 15 Lab: THE GAS LAWS 3. You have 2.70 +/- 0.05 kg of mass stacked on top of a rectangular column of gas with a movable, yet sealed top. The column is 2.0 cm by 2.0 cm at its base and 10.0 cm high. Uncertainty for length measurements is +/- 0.1 cm. The atmospheric pressure is 1.00 atm. a. Find the absolute pressure on the gas. Show your work. Check your units. 𝐹 𝐴 2.70 𝑘𝑔 ∗ 9.8𝑠𝑚2 𝑃 = 𝑃𝑎𝑡𝑚 + 𝑃 = 101300 𝑃𝑎 + 0.02 𝑚 ∗ 0.02 𝑚 168000 𝑃𝑎 b. Find the uncertainty in the area calculation for the situation given. Show your work. Max area with uncertainty in sides 4.4 E -4 Average (Regular) Area 4.0 E -4 Min area with uncertainty in sides 3.6 E -4 Uncertainty for area is 0.4 E -4 m2 c. Find the uncertainty in the gauge pressure for the situation given. Show your work. Max gauge P with uncertainty in mass and area Average (Regular) Area Min gauge P with uncertainty in mass and area 7.47 E 4 6.62 E 4 5.89 E 4 Uncertainty for gauge pressure is +/- 8500 Pa Physics 122 Lab: The Gas Laws Eyres page 2 rev: 10/11