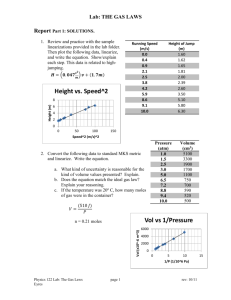
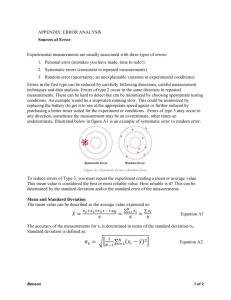
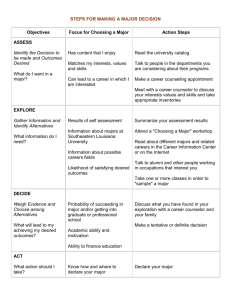
3. conclusion and evaluation labs(ce)
advertisement

IB Chemistry – INTERNAL ASSESSMENT GUIDELINES C. CONCLUSION AND EVALUATION (CE) ASPECT 1: CONCLUDING - Make a clear statement of conclusion (and the percent difference you calculated) by using your results to answer the original aim. If applicable, discuss whether or not you think the experiment supports your hypothesis, or the subject material covered in class. If applicable, explain the outcome and compare the results with accepted data values. Consider the significance of systematic or random errors in justifying your conclusion, including the direction in which systematic errors might influence the results. “*Please note that science is unable to "prove" anything, so an experiment that measures forces, masses, and accelerations will never "prove" Newton's Second Law. We often try to "confirm" our physical descriptions of the world, however: your measurements might (and should!) confirm the validity of Newton's Second Law.” ASPECT 2: EVALUATING PROCEDURE(S) - Identify the strengths & weaknesses of your experimental design and method of the investigation, including the precision and accuracy of the measurements. Consider what assumptions you have made in the design. ASPECT 3: IMPROVING THE INVESTIGATION Use the weaknesses identified above to guide you in suggesting improvements. These should aim to address reducing random error, removing systematic error and obtaining greater control of variables. ‘More time” and ‘use more accurate equipment’ are not very helpful. Comparing and Properly Analyzing % Uncertainty and % Difference Values *Be sure to address the following in the CONCLUSION section of your IB Lab write-ups. a) WHEN% diff is LESS THAN % uncertainty: Example Calculated % uncertainty = 7.7% Calculated % difference = 5.7% - % diff is less than % uncertainty therefore experiment is in agreement with the accepted literature value. - Improvements to experiment should focus on factors related to PRECISION/RANDOM ERRORS. Which factor/variable contributed the most to the % difference? (e.g. relative(%) error was highest for temperature, therefore suggest the use of a more precise thermometer…one that reads to ± 0.01 °C, for example) -Improvement Example 2: In a calorimetry experiment, increasing ΔT will also decrease relative error (increase denominator = decrease R.E.) *How increase ΔT?…increase concentration of reactants. -decreasing the RE of some measurements will be insignificant therefore little point in trying to decrease b) WHEN% diff is GREATER THAN % uncertainty: Example Calculated % uncertainty = 7.7% Calculated % difference = 23.5% - % diff is greater than % uncertainty therefore some major SYSTEMATIC ERRORS. Major focus of error analysis should therefore focus on identifying these. (e.g. heat loss to surroundings…not accounting for heat loss of calorimeter; specific heat of solution = specific heat of water assumption) A NOTE ON “HUMAN ERROR: PLEASE DON’T REFER TO “HUMAN ERROR”!! Examples of so-called human error include misreading a ruler, adding the wrong reagent to a reaction mixture, mis-timing the reaction, miscalculations, or any kind of MISTAKE. Scientists would never report the results of an experiment affected by human error -instead, they repeat the experiment more carefully! Points will be deducted from your lab report if you discuss "human error" instead of "experimental error." HUMAN ERROR = MISTAKE = REPEAT PROCEDURE AND ELIMINATE MISTAKE