Atomic Spectra Lab #4
advertisement

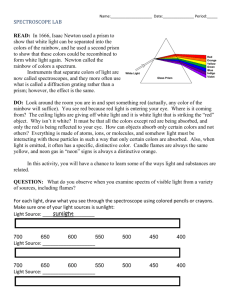
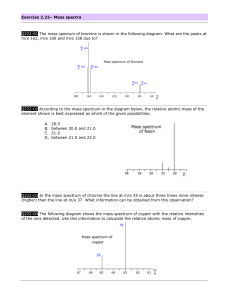
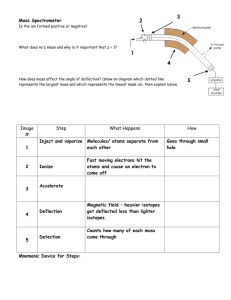
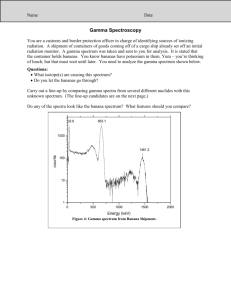
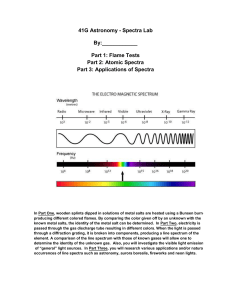
Atomic Spectra TBLS Chemistry Lab #4 Scenario: A sign company has three unknown samples of gases in glass tubing. They want to make a sign for a new restaurant opening using these colors. In order to place an order for new gases, however, they need to know what is in the tubes. Introduction: Sunlight passing though a prism produces a rainbow of colors – the visible spectrum. The separation of white light into its component colors occurs when light waves of different wavelengths are bent. When a pure atomic gas such as hydrogen or helium is subjected to a highvoltage electrical discharge, light is produced and the gas glows. When this light is passed through a prism however, the spectrum it produces is different. Instead of giving off a rainbow, the light emitted gives off a series of bright, colored lines called its atomic emission spectrum. Purpose: Use the line emission spectra produced by three elements to determine their identity. Procedure: 1. Using a spectroscope, observe the continuous “rainbow” spectrum from the sunlight. 2. Observe the atomic spectra of the three unknown gases once they have been inserted in to a power source. 3. After viewing each spectrum, record the number of lines seen for each color corresponding to the different wavelength ranges in the Raw Data table. 4. Using colored pencils, sketch the atomic spectra for each light source in the Processed Data table. 5. Repeat steps 4-6 after observing fluorescent light. Raw Data: Number of lines seen within each range of wavelengths, (nm) Light Source sunlight Fluorescent light Unknown 1 Unknown 2 Unknown 3 400-450 (purple) 450-500 (blue) 500-550 (green) 550-600 (yellow) 600-650 (orange) 650-700 (red) Total # of lines Processed Data: Light Source Atomic Spectra (sketch) I I I 400nm 500nm I I 600nm I I 700nm Sun Light Fluorescent Light Unknown 1 Unknown 2 Unknown 3 Conclusions: 1. What element does each unknown atomic spectrum most closely resemble? 2. Rank the colors in the visible spectrum from highest to lowest energy. 3. Do all of the colors of light span about the same width- do the bands of color appear equally wide or narrow? 4. What color in the spectrum appears brightest? Does this mean that is the highest energy? Why or why not? 5. Explain why violet light is released when an electron in a hydrogen atom falls from n=6 to n=2 while red light is released when an electron falls from n=3 to n=2. I