Reaction Rates and Chemical Equations - Varga
advertisement

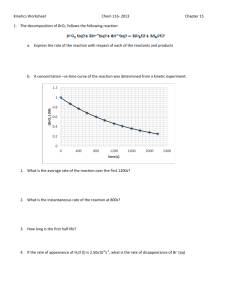
Chemistry 30 Reaction Rates and Chemical Equations We have learned that the speed of a chemical reaction can be determined by measuring the change in the concentration of amount of product / reactant as a function of time. The rate of a chemical reaction can be calculated in two different ways: Looking at the rate of appearance of products over a change in time Looking at the rate of disappearance of reactants over a change in time Examine the following chemical reaction, and notice how the molar ratio in the balanced chemical reaction plays a major role in the rate of formation of products / reactants: 2 HI(s) 1. When 2 moles decomposes then 2. When 1000 H2(g) + I2(g) 1. Reactions forms one mole of I2. 2. Reaction forms 500 molecules molecules of the decompose then product 3. When HI decomposes at 2.00 mol/L/s 4. If the rate of HI decomposition is 4.6 mol/L/s 3. Reactions forms I2 at 1.00 mol/L/s 4. Reactions forms I2 at 2.3 mol/L/s Chemistry 30 The Big Idea: The balances in the equation allow us to determine the relative rate of decomposition of reactants to the formation of products. Practice Question Examine the following reaction: N2 (g) + 3H2 (g) 2 NH3 (g) If the rate of decomposition of nitrogen gas is 0.03 mols per liter per second, what is the rate of formation of ammonia gas? Chemistry 30 Assignment: Balanced chemical equations and rate. *** If the rate of a reaction is known in terms of one particular substance in a reaction, the rate of the reaction in terms of the other reactants and products can also be determined. These relative rates can be expressed as ratios. Examine the following unbalanced reaction below. ___N2O5 (g) ___NO2 (g) + ___O2 (g) Balance the above reaction 1. Examine the mole ratios What is the relative rate of (expressed as a ratio) o o formation of O2 to the rate of decomposition of N2O5 ? formation of O2 to the rate of formation of N02? 2. If the rate of formation of O2 was 8.6 * 10 - 4 mol*L-1*s-1, what is the rate of decomposition of N2O5 ? 3. If the rate of formation of O2 was 4.3 * 10 - 4 mol*L-1*s-1, what is the rate of formation of N02 ? 3. In the following decomposition reaction, 2 N2O5 → 4 NO2 + O2 oxygen gas is produced at the average rate of 9.1 × 10-4 mol · L-1 · s-1. Over the same period, what is the average rate of the following: 4. the production of nitrogen dioxide the loss of nitrogen pentoxide Consider the following reaction: N2(g) + 3 H2(g) → 2 NH3(g) If the rate of loss of hydrogen gas is 0.03 mol · L-1· s-1, what is the rate of production of ammonia?