Gazi University
advertisement
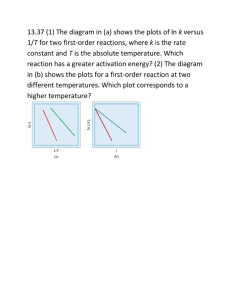
Gazi University Department of Chemical Engineering Che 341 Chemical Reaction Engineering 2008-2009 Fall Semester September, 2008 Homework-1 1) How much does a reaction rate with an activation energy of 14,700 cal/mole vary when the temperature is increased from 320 to 345 K? 2) Given that the first-order rate constant for the overall decomposition of N 2O5 is k=(4.3x1013 s-1) e-103 000/RT calculate the time required for 90% reaction at 50 0C. The activation energy is in J mol-1. 3) Although the thermal decomposition of ethyl bromide is complex, the overall rate is first order, and the rate constant is given by the expression k = (3.8x10 14 s-1) e -229 000/RT, where the activation energy is in J mol-1. Estimate the temperature at which the decomposition is 70% complete in 1 h. 4) A certain reaction has a rate given by ; -rA= 0.005 CA2, mol/ cm³.min If the concentration is to be expressed in mol/L and time in hours, calculate the rate constant in the units of moles, L and hour? 5) Milk is pasteurized if it is heated to 55 °C for 40 min., but if it is heated to 80 °C it needs 10 s for the same result. Find the activation energy (Ea) of this process? 6) The rate constant of a first order reaction is 4.60x10-4 s-1 at 350 °C. If the activation energy (Ea) is 104 kJ/mol , calculate the temperature at which its rate constant is 8.80x10-4 s-1 ? 7) Variation of the rate constant with temperature for the first order reaction : 2 N2O5 (g) 2 N2O4 (g) + O2 (g) is given in the following table. Determine graphically the activation energy for the reaction. T(K) k (s-1) 273 7.87x103 298 3.46x105 318 4.98x106 338 4.8707 8) Suppose that a substance X decomposes into A and B in parallel paths with rate constants given by kA = (1015 s-1) e-126 000/RT kB = (1013 s-1) e-83 700/RT where the activation energies are given in J mol-1. At what temperature will the two products be formed at the same rate?