HW 5-Spring 10
advertisement

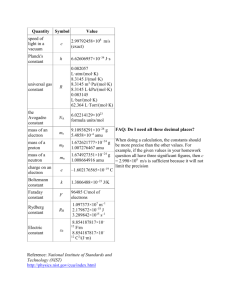
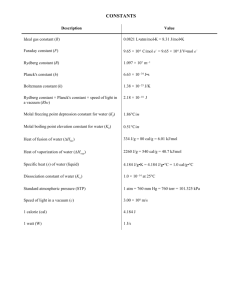
CHEN 354- HW 5—Due Friday, February 26, 2010 Problem 1. For the system ethylene(1)/propylene(2) as a gas, estimate fˆ1 , fˆ2 , ˆ1 , ˆ2 at T=150oC and P=30 bar and y1 = 0.35: (a) Through application of equation (11.63) (b) Assuming that the mixture is an ideal solution Problem 2. The data in Table 11.3 are experimental values of HE for binary liquid mixtures of 1,2 dichloroethane(1) and dimethyl carbonate (2) at 313.15 K and 1 atm. (a) Determine from the data numerical values of the parameters a, b, and c in the correlating equation: H E x1 x2 a bx1 cx12 (b) Determine from the results of part (a) the maximum value of HE. At what value of x1 does this occur? (c) Determine from the results of part (a) expressions for H1E and H 2E . Prepare a plot of these quantities vs. x1 and discuss its features. Problem 3. For an equimolar vapor mixture of propane (1) and n-pentane (2) at 75oC and 2 bar, estimate Z, HR and SR. Second virial coefficients, in cm3/mol are: T,oC 50 75 100 B11 -331 -276 -235 B22 -980 -809 -684 B12 -558 -466 -399 Equations (3.38), (6.55), (6.56), and (11.62) are useful. Problem 4. Laboratory A reports the following results for equimolar values of GE for liquid mixtures of benzene(1) with 1-hexanol(2): GE = 805 J/mol at T=298 K; GE = 785 J/mol at T =323 K Laboratory B reports the following result for the equimolar value of HE for the same system: HE = 1060 J/mol at T = 313 K Are the results from the two laboratories thermodynamically consistent with one another? Explain. Problem 5. Calculate and f by the Redlich-Kwong equation of state for acetylene at 325 K and 15 bar and compare with the results from a generalized correlation.